Short Communication
De-Novo Squamous Cell Carcinoma of Ovary - A Very Rare Tumor Masquerading as Uterine Fibroids
Pakhee Aggarwal*, Shweta Gangal, Suneeta Mittal
Department of Obstetrics & Gynaecology, Fortis Memorial Research Institute, Gurugram, India
*Corresponding author: Pakhee Aggarwal, Department of Obstetrics & Gynaecology, Fortis Memorial Research Institute, Gurugram, India
Published: 18 Feb, 2018
Cite this article as: Aggarwal P, Gangal S, Mittal S. De-
Novo Squamous Cell Carcinoma
of Ovary - A Very Rare Tumor
Masquerading as Uterine Fibroids. Clin
Oncol. 2018; 3: 1416.
Short Communication
Pure primary squamous cell carcinoma arising in the ovary is a very rare tumour. De-novo
occurrence of an ovarian squamous cell carcinoma (SCC) in absence of a primary ovarian dermoid
cyst or Brenner’s tumor occurs even less commonly than the incidence of SCC ovary which is about
2%-3% [1,2]. Some pure SCCs arise in association with a foci of endometriosis and very rarely
entirely de novo [1,2]. Mean age of occurrence of pure SCC is 56 years (27-73 years) [2]. Pure
primary SCC have been classified by the World Health Organization criteria as surface epithelialstromal
tumours [1] and are believed to arise from metaplasia of the surface epithelium of the ovary.
Till date less than 20 cases of de-novo SCC of ovary have been published [2,7].
A 56 year post-menopausal lady, P0L0 who was a known case of uterine fibroids presented to
the emergency with acute pain abdomen. She had a prior history of lower abdominal pain and fever
off and on for the past 4 months. Clinical examination was suggestive of an 18 weeks palpable mass
in lower abdomen with an irregular surface and restriced mobility. Her baseline haemogram, liver
and kidney functions were normal. Ultrasound was suggestive of an enlarged uterus with necrotic
areas likely fibroids, with multiple coarse interrupted calcifications along anterior wall of uterus. A
tubular structure either dilated fallopian tube or dilated appendix on right side of abdomen fused
with lower pole of kidney (cross fused ectopic kidney). Bilateral ovaries were not visualized. At this
point tumor markers were sent which showed mildly raised CA 125 and raised CEA with the values
as follows; CA 125:126.3, HE4:213, ROMA value: 73.70, CEA:>100. With a provisional diagnosis of
degenerated fibroids, patient was posted for laparotomy followed by pan-hysterectomy.
At laparotomy, a large mass about 18 weeks gestation uterine size was seen with partly mucoid
degenerative material and partly solid component. Uterus, bilateral tubes and ovaries were difficult
to deliniate separately from the mass. Mass was adherent above to the omentum and bowel loops,
laterally to pelvic side wall and anteriorly to bladder. Peritoneal fluid was collected for cytology. Both
ureters were traced. Both kidneys were fused together on right side. Sigmoid colon was adherent to
the tumor. Adhesiolysis was done and mass was separated all around from its attachments. In view
of the above findings, a frozen section biopsy from the mass was sent. This revealed features of a
squamous cell carcinoma probably arising in the ovary. Pan-hysterectomy with omentectomy was
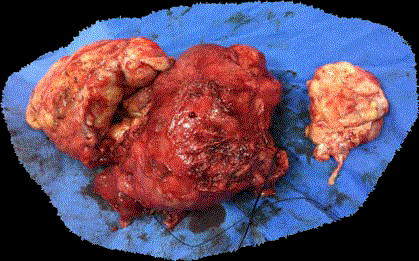
done following this report. On cut-section, the tumor was firm at places and necrotic at others (Fig
1). Patient made full recovery in the post-operative period and was discharged on 4th post-operative
day.
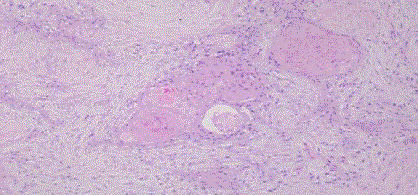
The final histopathology revealed a well to moderately differentiated squamous cell carcinoma –
possibly arising from a mucinous cystic tumor of endocervical type in the right ovary. There was no
evidence of endometriosis, Brenner’s tumor or any other ecto /meso/endodermal elements and no
normal ovarian tissue (Fig 2,3). The omentum was free of tumor. Peritoneal fluid cytology showed
nucleated and anucleated squamous epithelial cells in a background of inflammatory cells, RBCs
and debris; with a few squamous cells having atypia. Based on the staging, she was diagnosed as
well to moderately differentiated squamous cell carcinoma of right ovary arising de novo, stage IIC.
SCC of the ovary are very aggressive tumors, presenting as symptomatic abdominal mass,
requiring histopathology for final diagnosis and a multimodality treatment approach. Treatment
needs to be structured as per the patient’s clinical status [2,5]. Frozen section has a role in the
diagnosis of epithelial or other category of tumours since optimal cytoreduction is the primary
management for epithelial tumours [5].
The role of elevated Ca-125 in de novo SCC has not been very well established and CA 125 can
be normal to mildly elevated [8] as in this patient. Pure SCC have been found to be positive for IHC
markers -MA903, p63 and ck 5/6.
The data on post operative adjuvant therapy, either radiotherapy,
chemotherapy or both are insufficient. Chemotherapy with
platinum based agents has shown variable results [4,7,9,10]. SCC is
a radiosensitive tumour and concurrent weekly chemotherapy with
cisplatin and whole pelvis radiation has shown benefit, extrapolated
from results for SCC within antecedent dermoids [11]. However,
whether the same concept applies to de novo SCC is not clear.
In advance stages de-novo SCC behaves aggressively with
recurrences within 5 weeks to 6 months [4,7], in spite of initial
response to cytoreduction and chemotherapy. Overall survival in denovo
SCC ranges from 5 weeks to 24 months [4,7,10].
This patient underwent optimal debulking and was referred for
post-operative radiation but was lost to follow up after 5 months of
surgery.
Thus, albiet an uncommon tumor, pure primary SCC of the ovary
can mimic other benign conditions and confuse the clinical picture.
It is important to have a good pathology backup when trying to
diagnose this rare tumor, whose incidence seems to be rising and a
multidisciplinary management approach to maximise survival.
Figure 1
Figure 2
References
- Clement PB, Young RH, et al. Ovarian surface epithelial-stromal tumors. In: Mills SE, Carter D, Greenson JK, et al., editors. Sternberg’s diagnostic surgical pathology. 5. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 2278–2341.
- Pins MR, Young RH, Daly WJ. Primary squamous cell carcinoma of the ovary. Report of 37 cases. Am J Surg Pathol. 1996;20(7):823-33.
- Chien SC, Sheu BC, Chang WC, Wu Mz, Huang SC. Pure primary squamous cell carcinoma of the ovary: a case report and review of the literature. Acta Obstet Gynecol Scand. 2005;84(7):706-8.
- Todo Y, Minobe S, Sasaki M, Yamamoto R, Sakuragi N. A case of pure-type ovarian squamous cell carcinoma for which combination chemotherapy consisting of paclitaxel and carboplatin was not effective. Gynecol Oncol. 2005;97(1):223-7.
- Park J-Y, Song JS, Choi G, Kim JH, Nam JH. Pure primary squamous cell carcinoma of the ovary: a report of two cases and review of the literature. Int J Gynecol Pathol. 2010;29(4):328-34.
- Mahe E, Sur M. Primary squamous cell carcinoma of the ovary. MUMJ 2011;8(1):80-3.
- Amjad AI, Pal I. De novo primary squamous cell carcinoma of the ovary: A case of a rare malignancy with an aggressive clinical course. J Pak Med Assoc. 2008;58(5):272-4.
- Acien P, Abad M, Mayol MJ, Garcia S, Garde J. Primary squamous cell carcinoma of the ovary associated with endometriosis. Int J Gynecol Obstet. 2010;108|(1):16-20.
- Eltabbakh GH, Hempling RE, Recio FO, O’Neill CP. Remarkable response of primary squamous cell carcinoma of the ovary to paclitaxel and cisplatin. Obstet Gynecol. 1998;91(5):844-6.
- Ohtani K, Sakamoto H, Masaoka N, Nagai N, Satoh K, Kanaeda T, et al. A case of rapidly growing ovarian squamous cell carcinoma successfully controlled by weekly paclitaxel-carboplatin administration. Gynecol Oncol. 2000;79(3):515-8.
- Dos Santos L, Mok E, Iasonos A, Park K, Soslow RA, Aghajanian C, et al. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: a case series and review of the literature. Gynecol Oncol. 2007;105(2):321-4.