Clinical Image
Inhibition of Chronic Osteomyelitis using Sustained Release of Drug from Biodegradable Polymeric Chip
Pralay Maiti1*, Sudipta Senapati1 and Shyam K Saraf2*
1School of Materials Science and Technology, Indian Institute of Technology (Banaras Hindu University), India
2Department of Orthopedics, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221 005, India
*Corresponding author: Pralay Maiti, Department of
Orthopedics, Indian Institute of
Technology (Banaras Hindu University),
Varanasi 221 005, India
Shyam K Saraf, Department of
Orthopedics, Institute of Medical
Sciences, Banaras Hindu University,
Varanasi 221 005, India
Published: 06 Jan, 2018
Cite this article as: Maiti P, Senapati S, Saraf SK.
Inhibition of Chronic Osteomyelitis
using Sustained Release of Drug from
Biodegradable Polymeric Chip. Clin
Oncol. 2018; 3: 1406.
Clinical Image
Despite the recent advances in medical and surgical therapies, the treatment of chronic
osteomyelitis (bone and joint infection), especially the treatment of Methicillin-Resistant
Staphylococcus Aureus (MRSA) osteomyelitis, still remains challenging and is associated with
high recurrence rate, morbidity and substantial healthcare cost [1,2]. Current treatment of chronic
osteomyelitis primarily involves the surgical debridement of diseased bone tissue followed by long
term systematic antibiotic therapy. Poly(methyl methacrylate) (PMMA) beads impregnated with
vancomycin, gentamicin or tobramycin have been used commonly for many years as main stream
local delivery vehicle in osteomyelitis treatment [3]. However, PMMA beads cannot be degraded or
absorbed in the body and it must be removed in a second operation along with its suboptimal release
profile with meager 25% to 50% of the antibiotic can be eluted in 4 weeks time, which is far away
from being a controlled antibiotic drug delivery carrier [4]. Biodegradable polymer based devices
can provide a more controlled release profile and eliminate the utmost need for the additional
(removal) surgery of conventional carrier. An ideal antibiotic vehicle should be such that it can
improve the therapeutic efficacy of the antibiotic drug and provide a sustained but prolonged release
at the site of infection without any local or systematic toxicity while it should be bioadsorbable
[5-7]. Recently, we have developed vancomycin loaded biodegradable polycaprolactone (PCL)
chip as a local antibiotic drug delivery vehicle for artificially MRSA-infected osteomyelitis which
shed light on the new treatment of bone infection without second surgery to remove implant [8].
Although vancomycin is considered as an effective antibiotic against MRSA, sufficient dose of pure
vancomycin is unable to penetrate efficiently into local sites due to the growth of malformations
nearing the infection sites. The advantage of local antibiotics delivery is to provide a high drug
concentration at the infection site for a prolonged period in sustained manner without systemic
side effects.
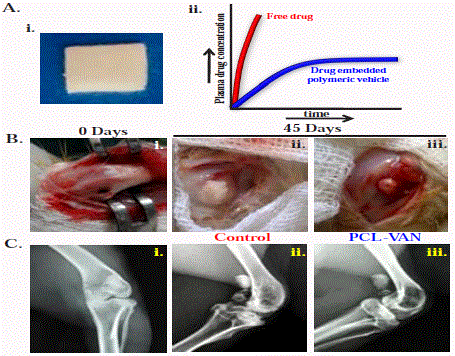
Vancomycin embedded in a polymer chip (5% (w/w) of vancomycin with respect to polymer) has
been developed through solution route, showing sustained release of
vancomycin from the chip (Figure 1A). Vancomycin, as an antibiotic,
is characterized through MIC in the range of 0.5 to 2 μg ml-1 against
the MRSA strain [8]. The MIC of the PCL-VAN chip (vancomycin
embedded in PCL) is measured to be 1.98 μg ml-1 which suggests that
the antimicrobial activity against MRSA can well be maintained using
PCL-VAN chip. A unicortical defect was created in the metaphysis
of the distal femur of healthy male rabbits. A mixed strain of MRSA
obtained from a patient suffering from chronic osteomyelitis has
been used to induce osteomyelitis at the defect. Rabbits were divided
into two groups: Group I (Control) received only free vancomycin
while defects in group II were filled with PCL-VAN chips (Figure
1B). Local signs of infection with discharge of pus are observed
in all the rabbits of control group. In contrast, suture lines of the
limbs are healthy and dry in all the rabbits treated with PCL-VAN
chips. Radiographs of control group rabbits exhibit new abscesses,
profound cortical reactions and typical signs of osteomyelitis such as
osteolysis, sclerosis and sequestrum (Figure 1C). No sign of infection
is observed in PCL-VAN treated group instead healthy and recovered
bone is noticed clearly suggesting the efficacy of the sustained release
along with the bioadsorbability of the chip through biodegradation
as required for bone growth. This result illustrates the usefulness of
PCL-VAN chip which release the antibiotics within therapeutic levels
over a prolonged period and thereby stimulating the antimicrobial
activity.
Figure 1
Figure 1
(A) (i) Photographic image of the developed antibiotic carrier (PCL-VAN chip); (ii) Schematics of
plasma drug concentration vs. time profile showing burst release for free drug and sustained release from
the chip. (B) Photographic images of the surgical site, (i) creation of a unicortical metaphyseal defect in distal
femur. Clinical photograph showing (ii) severe infection (white pus) in the control group against (ii) healthy bone
formation in the PCL-VAN treated group. (C) Radiographic images showing (i) normal healthy rabbit limb at the
beginning of the experiment (ii) control group having severe infected bone and (iii) healthy bone treated with
PCL-VAN chip after indicated time (45 days).
References
- A Hogan, VG Heppert, AJ Suda, Osteomyelitis, Arch. Orthop. Trauma Surg. 2013;133(9):1183-96.
- Jiang JL, Li YF, Fang TL, Zhou J, Li XL, Wang YC, et al. Vancomycinloaded nano-hydroxyapatite pellets to treat MRSA-induced chronic osteomyelitis with bone defect in rabbits. Inflammation research. 2012; 61(3):207-15
- Hanssen AD, Spangehl MJ. Practical applications of antibiotic loaded bone cement for treatment of infected joint replacement. Clin Orthop. 2004;1(427):79–85.
- Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am. 2004;86-A(10): 2305–18.
- Mahanta AK, Senapati S, Maiti P. A polyurethane chitosan brush as an injectable hydrogel for controlled drug delivery and tissue engineering. Polym Chem. 2017;10(40).
- Senapati S, Thakur R, Verma SP, Duggal S, Mishra DP, Das P, et al. Layered double hydroxides as effective carrier for anticancer drugs and tailoring of release rate through interlayer anions. J Control Release. 2016; 224:186- 198.
- Kumar S, Singh S, Senapati S, Singh AP, Ray B, Maiti P. Controlled drug release through regulated biodegradation of poly (lactic acid) using inorganic salts. Int J Biol Macromol. 2017;104(pt A):487-497.
- Rai A, Senapati S, Saraf SK, Maiti P. Biodegradable poly (ε-caprolactone) as a controlled drug delivery vehicle of vancomycin for the treatment of MRSA infection. J Mater Chem B. 2016; 4(30): 5151-60.