Case Report
Cutaneous Nodules Revealing Large Cell Anaplastic Lymphoma ALK+
Omahsan L1*, Zerrouki N1, Bazouti S1, Zerouati Erouati K3, Benajiba N3, Zizi N1,2, Dikhaye S1,2
1Department of Dermatology, Mohammed VI University Hospital, Morocco
2Department of Epidemiology, Laboratory for Scientific Research and Public Health, Morocco
3Department of Pediatry, Mohammed VI University Hospital, Morocco
*Corresponding author: Omahsan L, Department of Dermatology, Mohammed VI University Hospital, Morocco
Published: 02 Jan, 2018
Cite this article as: Omahsan L, Zerrouki N, Bazouti S,
Zerouati Erouati K, Benajiba N, Zizi N,
et al. Cutaneous Nodules Revealing
Large Cell Anaplastic Lymphoma ALK+.
Clin Oncol. 2018; 3: 1402.
Abstract
Anaplastic Large Cell Lymphoma (ALCL) encompasses at least 3 clinically distinct entities. The
entity ALK with systemic ALCL is rare. In the infant population skin involvement is rarely revealing.
Diagnosis and early management improves prognosis. We report an original case of a 7-year-old
child in whom the skin involvement was indicative of neoplasia.
Keywords: Cutaneous nodules-large cell anaplastique lymphoma ALK+ -chemotherapy
Introduction
Anaplastic large cell lymphoma (ALCL) is an uncommon T-cell lymphoma defined according to the Revised European American Lymphoma and World Health Organization classifications as a distinct clinicopathological entity [1]. Cutaneous ALCL presents either as primary cutaneous disease or as secondary skin involvement due to the systemic disease. We describe an unusual systemic ALCL revealed by cutaneous nodules.
Case Presentation
A 7-year-old child with a history of herpetic encephalitis at 2 months of age, consult the
emergency room for nodal lesions that have been evolving for 3 weeks with anaphylaxis. The
examination of the skin revealed numerous nodular swellings which were erythematous-violin, not
painful and slightly pruriginous. Some of which had a surface ulcerated, and others were covered
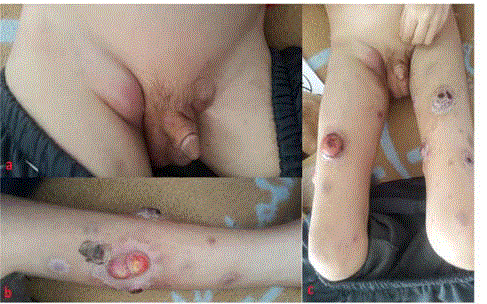
by blackened crusts, sitting in the lower limbs, scalp and in left orbital (Figure 1). Adding to this,
he had some erythematous and finely scaly macules in the trunk and back. The examination of the
ganglionic areas found multiple cervical, spinal and inguinal nodes (Figure 1), the largest of which
measured 3cm of major axis sitting at the right inguinal level, fixed with erythematous opposite
surface.
The biological examinations revealed a normochromic normocytic argenerative anemia at 5g/
dl, without leucopenia or thrombocytopenia with. Renal and hepatic imaging was not abnormal.
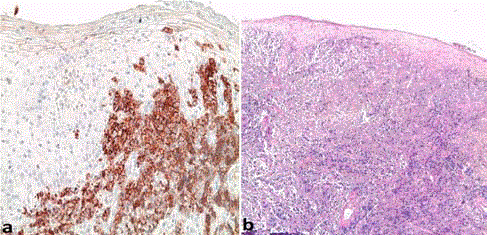
The cutaneous biopsy with immunohistochemical study confirmed the diagnosis of a cutaneous
localization of malignant non-Hodgkin lymphoma with large ALK + anaplastic cells (Figure 2).
The osteomedullary biopsy revealed a medullary invasion of a malignant tumor process with little
differentiation. The extension report showed only tumor-like gonglions. The evolution was marked
by a subsidence of the nodules after the 3rd cure of chemotherapy, CHOP protocol. Unfortunately
the child died at the 6th cure by a sepsis.
Discussion
Anaplastic Large Cell Lymphoma (ALCL) was first described in 1985 based on the large pleomorphic cells expressing CD30, its propensity to invade sinusoids, and the cohesive appearance of the tumor [2]. ALCL encompasses at least 3 clinically distinct entities (primary cutaneous ALCL (pcALCL), anaplastic lymphoma kinase (ALK) with systemic ALCL (sALCL), and ALK without sALCL [3,4]. sALCL is usually positive for ALK and epithelial membrane antigen (EMA) expression; which are negative in pcALCL [3]. Our patient had CD30+, ALK+ and EMA+ with medullary invasion by tumoral cells. sALCL are known to be in advanced-stage disease at presentation. It primarily involves lymphnodes, although extranodal sites maybe involved [5]. In children, 18–25% of ALCLs develop skin manifestations during the course of the disease [6]. These manifestations rarely reveal the disease. Our patient had already developed cutaneous nodules 3 weeks previously. The histopathology study allowed making the diagnosis. In children, the systemic form is more common and prognosis is worst when the skin is also affected [7,8]. sALCL are treated with chemotherapy containing antrhracycline such as CHOP [9]. Autologous and allogeneic stem cell transplantation may be beneficial in case of recurrence [9]. Brentuximab vedotin is an anti-CD30 monoclonal antibody targeting malignant CD30-positive cells, which shows also its efficiency in the treatment of refractory and recurrent systemic ALCL [10]. The decision was to treat our patient with CHOP protocol with a good improvement from the 3rd cure of chemotherapy.
Figure 1
Figure 1
A: clinical aspect of bilateral inguinal adenopathies. B and C:
multiple cutaneous nodules of the lower limbs.
Figure 2
Conclusion
We report an original pediatric case of sALCL AKL+. The cutaneous biopsy plays an important role in making this diagnosis. Early detection and treatment may provide a better outcome.
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC. 2008;312-6.
- Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848-58.
- Alsayyah A, AlHumidi A. A primary systemic ALCL present initially as cutaneous localized skin lesions: report of an unusual case. Am J Dermatopathol. 2015;37(1):e12-e4.
- Savage KJ1, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496-504.
- Kim KH1, Jung YH1, Han CW2, Woo IS3, Son JH3. A case of Primary Bone Anaplastic Large Cell Lymphoma. Am J Case Rep. 2016;17:734-738.
- Seidemann K1, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 2001;97(12):3699-706.
- Hung TY1, Lin YC, Sun HL, Liu MC. Primary cutaneous anaplastic large cell lymphoma in a young child. Eur J Pediatr. 2008;167(1):111-3.
- Le Deley MC1, Reiter A, Williams D, Delsol G, Oschlies I, McCarthy K, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008;111(3):1560-6.
- WilliWoessmann, Martin Zimmermann, MeikeLenhard et al. Anaplastic Large-Cell Lymphoma in Children and Adolescents after Berlin-Frankfurt-Muenster (BFM)–Type First-Line Therapy: A BFM-Group Study. Journal of clinical oncology. VOLUME 29,NUMBER 22, AUGUST -1- 2011.
- Xueyan Chen, Lorinda A Soma, and Jonathan R Fromm. Targeted therapy for Hodgkin lymphoma and systemic anaplastic large cell lymphoma: focus on brentuximabvedotin. Onco Targets Ther. 2014;7:45-56.