Case Report
Repeated Isolated Limb Infusion as Limb Salvage Therapy for Recurrent Unresectable Extremity Sarcoma
Leah E. Hendrick1, David Portnoy3, Michael Neel4, Martin D. Fleming1,2 and Jeremiah L. Deneve DO1,2*
1Department of Surgery, University of Tennessee Health Science Center, USA
2Department of Surgical Oncology, University of Tennessee Health Science Center, USA
3Department of West Cancer Center, University of Tennessee Health Science Center, USA
4Ortho Memphis, Memphis, TN, USA
*Corresponding author: Jeremiah L. Deneve, Department of Surgical Oncology, University of Tennessee Health Sciences Center, 910 Madison Avenue, Suite 303, Memphis, TN 38163, USA
Published: 19 Jan, 2018
Cite this article as: Hendrick LE, Portnoy D, Neel M,
Fleming MD, Jeremiah L Deneve DO.
Repeated Isolated Limb Infusion as
Limb Salvage Therapy for Recurrent
Unresectable Extremity Sarcoma. Clin
Oncol. 2018; 3: 1400.
Abstract
Introduction: Recurrent Soft Tissue Sarcoma (STS) of the extremity remains a difficult challenge.
Isolated Limb Infusion (ILI) is a treatment option for patients with unrespectable extremity STS who
would otherwise require amputation. We present a case of recurrent extremity sarcoma managed
with ILI as a limb-salvage alternative to amputation.
Methods: We present a case of recurrent extremity sarcoma managed with repeated ILI and a brief
review of the literature.
Case Report: A 34 year old male presented in July 2013 as a referral for forequarter amputation
for management of unresectable recurrent left upper extremity sarcoma. At initial presentation
in 2011, the tumor involved the median nerve. Neoadjuvant radiation was administered and a
microscopic margin positive resection of a single left forearm lesion was performed. The patient
developed multifocal recurrence in 2013 with lesions involving the initial left forearm site, the left
upper extremity along the biceps tendon, a left clavicular lesion and left axillary lymphadenopathy.
CT imaging of the chest demonstrated no evidence of distant disease. Resection of the multiple
recurrences was performed followed by ILI. A partial response to ILI was initially observed.
Additional disease developed 9 months after ILI and repeat ILI was performed. Forequarter
amputation was ultimately required for local control of additional multifocal recurrence, 16 months
after initial ILI. Distant disease eventually developed to which the patient succumbed in late 2015.
Conclusion: Management of recurrent extremity STS remains a difficult challenge. Repeated ILI
offers an alternative to amputation for selected patients while maintaining function and quality of
life.
Keywords: Sarcoma; Isolated Limb Infusion; Weiberdink; Recurrence; Amputation
Background
Soft Tissue Sarcomas (STS) are a heterogenous group of malignancies with distinct clinical and pathologic features [1]. STS are relatively rare, accounting for only 1% of adult malignancies with an estimated 11,900 people diagnosed annually in the United States [2]. More than 50 different subtypes have been identified with undifferentiated pleomorphic sarcoma, liposarcoma, leiomyosarcoma, synovial sarcoma and malignant peripheral nerve sheath tumors being most common [3]. These tumors may arise from the extremity, head/neck and truncal region and retroperitoneum or chest wall. Several prognostic factors such as tumor stage, size, grade and anatomic location have been demonstrated to have an impact on overall survival [4-6] with margin of resection, histology and the use of radiation also affecting local disease control [7-9]. Surgery is the standard treatment for primary extremity STS. A microscopic negative margin resection may be difficult in situations of abutment or involvement of critical neurovascular structures. In these situations, Radiation Therapy (RT) may be administered as either neoadjuvant therapy or in the adjuvant setting. Positive margins after surgical resection are associated with an increased risk of local recurrence [10,11]. Re-resection to negative margins is preferred, but may not be possible, especially in situations of critical nerve or vascular involvement or when resection may result in loss of function or significant impairment of the involved extremity. Postoperative RT is recommended and has been demonstrated to improve the local control of patients with positive surgical margins [12,13]. Despite appropriate aggressive multimodality therapy, local recurrence is common, affecting up to 7-24% of cases [11,14,15]. Management of recurrent STS is a challenge as often times many patients have undergone neoadjuvant therapy prior to surgical resection. Local recurrence for those who may not have undergone preoperative therapy may be associated with a better prognosis than for those treated with a combined multimodality initial approach [16]. Little is known about the effect of local recurrence on overall outcome. It is generally thought that local recurrence of STS is associated with a poor prognosis and has a negative impact on distant metastasis and survival [17]. Historically, local recurrences were managed with extremity amputation [18]. Amputation offers definitive local control but does so often at the expense of patient function with the potential for significant morbidity and negative impact on quality of life. Furthermore, amputation does not have an impact on distant metastasis or overall outcome [19-21]. For this reason many suggest that attempts to surgically manage local recurrence a second time should be more aggressive than the initial treatment approach. Re-resection of extremity STS recurrence is the preferred treatment option if possible, as well as the repeated use of irradiation for those who received radiation during initial management. Both of these treatment approaches, however, are associated with a high-risk of treatment-related morbidity and may negatively affect extremity function. Selecting the most appropriate therapy that allows a patient to maintain function and quality of life without negatively affecting long-term outcome is important. The regional administration of chemotherapy in the form of hyperthermic isolated limb perfusion (HILP) or Isolated Limb Infusion (ILI) allows treatment of the recurrent disease while sparing the need for amputation and maintaining extremity function and quality of life. Both procedures can be performed as either primary treatment of recurrent disease or as adjuvant treatment combined with resection and irradiation. We present a case of multifocal recurrent extremity STS managed with resection and repeated ILI as an alternative to amputation. A brief review of the literature focusing on the management of locally recurrent STS of the extremity with ILI is discussed.
Table 1
Table 2
Figure 1
Figure 2
Case Presentation
A 34 year old male presented in July 2013 as a referral for
forequarter amputation for management of unresectable recurrent left
upper extremity sarcoma. At the time of his initial presentation, the
tumor was found to involve the median nerve. Neoadjuvant radiation
therapy was administered and a microscopic margin positive resection
was performed in 2011 of a single left forearm lesion. The following
year, the patient developed left axillary lymphadenopathy. Excisional
biopsy was performed which demonstrated metastatic high-grade
sarcoma. He was treated with Adriamycin and iphosphamide.
The patient developed multifocal recurrence in 2013 with lesions
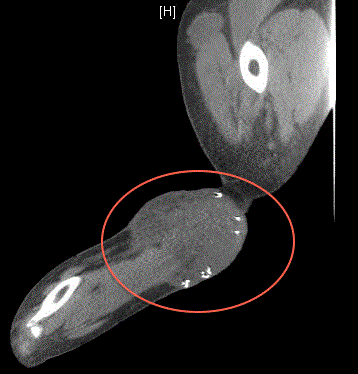
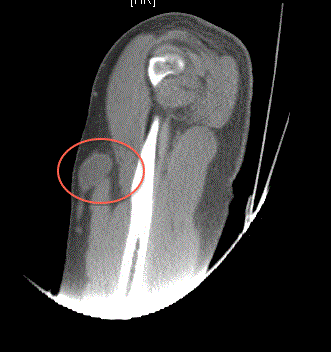
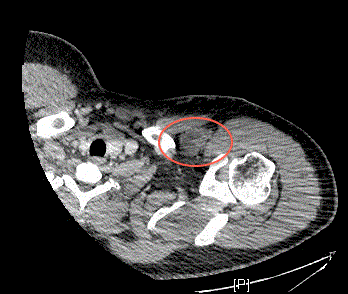
involving in the left forearm at the site of initial resection (Figure
1), the left upper extremity along the biceps tendon (Figure 2), a left
clavicular lesion (Figure 3) and left axillary lymphadenopathy. CT
imaging of the chest demonstrated no evidence of distant disease.
Forequarter amputation was recommended for local control of the
multifocal recurrence, which the patient subsequently declined. He
was referred for evaluation for palliative ILI.
Resection of the proximal disease was performed including the
left clavicular and bicep lesions as well as left axillary lymph node
dissection. The forearm lesion was deemed unresectable because of
median nerve involvement and left in situ. At the same operation,
following resection left upper extremity ILI was performed using
melphalan and Actinomycin D. Final pathology demonstrated
high-grade sarcoma with negative margins from the left clavicular
and biceps lesions. The left axillary lymph node dissection revealed
metastatic high-grade sarcoma involving 2 of 27 lymph nodes.
Postoperatively, the patient was monitored in the intensive care unit,
where serial Creatinine Phosphokinase (CPK) levels were followed.
After the CPK peaked and began to trend down to normal, he was
discharged home (postoperative day 3). He tolerated ILI well with
no serious side effects (Weiberdink I-II). Four months post-ILI, a
partial clinical response of the left forearm lesion was observed with
a notable decrease in size. Approximately 9 months after initial ILI, a
new left shoulder mass developed and the left forearm lesion began to
increase in size. Amputation versus resection and repeated ILI were
presented as treatment option. Repeat resection of the left shoulder
disease was performed followed by repeated ILI to manage the left
forearm lesion.
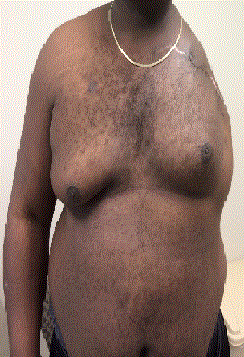
Disease rapidly recurred involving the left shoulder girdle 3
months after repeated resection/repeat ILI and forequarter amputation
was eventually performed in November 2014, 16 months after initial
ILI (Figure 4). Repeated ILI and resection spared a functionally
debilitating forequarter amputation for almost a year and a half. The
high grade STS continued to progress, however, and CT imaging of
the chest in May 2015 demonstrated multiple pulmonary metastases.
The patient eventually died of disease progression in late 2015.
Figure 3
Discussion
This case highlights some of the difficulties experienced in managing patients with local recurrence of extremity STS. As in this case presented, involvement of critical neurovascular structures may limit treatment options and/or resectability and have a negative impact on local control. The development of multifocal or distant disease, which may occur synchronously at the time of recurrence, affects treatment planning and recommendation. Obtaining local control in the setting of distant disease certainly becomes more challenging. Often, treatment efforts focus on distant metastases and the local disease process is left untreated unless symptomatic. How to appropriately manage multifocal recurrence and/or symptomatic local recurrence is difficult. For patients with bulky recurrent tumors involving critical neurovascular structures, selecting an appropriate therapy that provides local control without compromising quality of life, limb function or long-term outcome is a challenge. Amputation, as previously mentioned, provides definitive local control, but at the expense of loss of function and does nothing to address neither distant disease nor does it impact overall survival [19,22]. There has been a growing trend over the last several decades toward limbpreservation therapies when treating these complicated patients. Regional delivery of chemotherapy can be used as an adjunct to surgical resection with radiation therapy for advanced STS of the extremity. Furthermore, regional therapy offers the potential for local control, especially in situations of unresectable, isolated recurrent disease or recurrent disease in the presence of distant metastasis. Two techniques, HILP and ILI, allow the regional administration of chemotherapy, delivering drug concentrations 15 to 25 times higher than systemic dosages without the systemic side effects [23]. There are numerous potential indications for regional therapy for STS, most notably: multifocal primary tumors [24] recurrent disease [25] those undergoing prior resection with irradiation [26] bulky primary tumors [27,28] or high-grade tumors [27]. Elderly patients [29] and those with distant metastasis treated in a palliative setting [30] have been shown to gain a benefit in local control and limb-salvage rates using these techniques. HILP was initially described in the 1950s for treating melanoma patients with regional in-transit disease and was the first regional chemoperfusion therapy to demonstrate an improvement in limb-salvage rates in patients with unresectable extremity STS [23] HILP is an invasive technique and requires an extracorporeal bypass circuit [31]. ILI is a less invasive approach for administering regional chemotherapy, which was first described in the 1990’s by the Sydney Melanoma Unit for the treatment of intransit metastases for melanoma [32]. ILI is technically a less complex procedure with overall response rates close to those observed with conventional HILP.
Figure 4
Procedural Details
ILI is performed under hyperthermic, nonoxygenated conditions
using percutaneously placed catheters. High-flow 5Fr to 6Fr arterial
and 6Fr to 8Fr venous catheters are inserted by way of an uninvolved
lower extremity and advanced fluoroscopically into the involved
extremity. This may be done pre-operatively by interventional
radiology or intraoperatively by vascular surgery after the induction
of general anesthesia. Heparin is administered to achieve full systemic
anticoagulation with a target activated clotting time greater than or
equal to 350 seconds. The catheters are connected to an infusion
circuit that consists of a heat exchanger and bubble excluder. Once
subcutaneous temperatures of greater than 37°C are achieved, a
pneumatic tourniquet is placed on the proximal aspect of the limb
to be infused, isolating the limb from the systemic circulation.
Papavarine (60 mg) is injected into the arterial catheter and
chemoperfusion is initiated. The cytotoxic agents, often melphalan
and acitnomycin D, are administered and circulated for 30 minutes.
After infusion is complete, the limb is manually flushed with one
liter of isotonic crystalloid solution, heparinization is reversed with
protamine and the catheters are removed when the ACT is at or near
baseline. Patients are monitored daily for regional toxicity with serial
Creatine Phosphokinase (CPK) measurements and discharged home
when CPK levels peak and fall back towards baseline, generally within
4 to 6 days [33].
Toxicity of ILI
ILI is generally well tolerated with mild side effects and systemic
toxicity is uncommon. Rhabdomyolysis, as measured by serum CPK,
may be encountered in patients undergoing ILI, therefore patients are
monitored in the hospital for several days post-treatment. CPK levels
often rise greater than 1000 IU/L and require daily blood urea nitrogen
and creatinine level monitoring to avoid myoglobin-induced renal
failure. Aggressive hydration with isotonic saline and maintenance
of urine output greater than 0.5 mL/kg/hr may reduce the risk of
renal failure caused by myoglobinuria [34]. Patients with CPK levels
greater than 1000 IU/L are hydrated and given corticosteroids to
decrease muscle edema and inflammation and are monitored until
CPK levels fall towards baseline.
Regional toxicity is more commonly observed with ILI
than systemic toxicity. A commonly used classification system
characterizing acute tissue reactions was first reported by Wieberdink
and has standardized regional toxicity reporting for patients
undergoing ILI therapy (Table 1) [35]. Acute regional toxicity-related
symptoms are typically observed within the first 72 to 96 hours after
ILI [32,36]. Common reactions to therapy include mild erythema,
edema and pain (Wieberdink II-III). Mild to moderate blistering is
frequently seen up to several days’ post-ILI therapy. The development
of compartment syndrome progressing to the need for amputation
has been reported but is generally uncommon. Long-term toxicity
is infrequently reported and may include parathesias or permanent
nerve dysfunction.
Results of ILI
The outcome for patients undergoing regional therapy with ILI
for extremity STS is generally favorable. Most patients experience
at least a partial response to therapy which is assessed generally
3 months after ILI treatment. Disease responses are assessed by a
combination of physical examination and cross-sectional imaging
andare characterized as: complete response, partial response,
stable disease and no response. Overall response rates are similar
for those undergoing ILI or HILP but HILP is thought to produce
more complete responders than ILI. Tumor response rates range
from 79% to 90% for patients with advanced extremity STS that
undergo ILI who were previously only considered for amputation
[24,33,37]. The outcome for patients treated with ILI for extremity
STS is listed in (Table 2). Hegazy and colleagues first reported on
40 patients with unresectable extremity STS who were treated with
ILI using doxorubicin [24]. All patients were felt to be unresectable
at initial diagnosis, and amputation was considered as the only
viable treatment option. ILI was performed pre-operatively with
doxorubicin (0.7 mg/kg for upper extremity or 1.4 mg/kg for lower
extremity) followed by external beam radiotherapy (started 3-7
days after ILI) for a total dose of 35 Gy in ten fractions. An objective
clinical tumor response was observed in 85% of patients and the
sarcoma was rendered resectable in most cases. With a median follow
up of 15 months, a limb salvage rate of 82.5% was achieved using this
multimodality treatment regimen. Turaga and colleagues reported
similar results in 2011 with a series of 22 patients with non-melanoma
cutaneous metastases managed with ILI for limb threatening disease
[33]. Fourteen of the 22 patients (64%) underwent ILI for STS.
Overall end points included limb preservation and clinical tumor
response. Two patients underwent repeat ILI (1-Kaposi sarcoma,
1-pleomorphic high-grade sarcoma). The response rate for patients
with STS was 75% (17% complete response and 58% partial response)
per patient and 78% (14% complete response and 64% partial
response) per infusion. Vohra and colleagues reported on a series
of 26 ILI procedures (19-lower extremity, 7-upper extremity) in 22
patients with locally advanced, unresectable STS [38]. The authors’
primary end points were in-field response and limb-salvage rates.
Seventeen patients were evaluable at 3 months of follow up. No grade
IV Wieberdink toxicity was observed. Four (24%) had a complete
response, 3 (18%) partial response, 3 (18%) stable disease and 7 (41%)
had disease progression. Twelve of the 17 evaluable patients were able
to undergo successful limb-salvage. Importantly, 2 (12%) were able to
undergo resection after ILI and remain free of disease afterwards for
22 and 30 months, respectively. In addition to tumor response, the
use of ILI may spare these complex patients the potential morbidity
and need for amputation. ILI limb-salvage rates are similar to those
observed with HILP. Four of the larger ILI series for STS document
limb-preservation rates in the range of 71% to 83% [24,33,38] Local
or regional recurrence after ILI has been reported and can occur in
42% to 48% depending on the prior treatment [24,37]. Repeat ILI in
these instances, offers additional treatment of the tumor and local
control, even in the presence of distant disease.
Conclusion
Local recurrence of extremity STS remains a challenging problem to treat. Resection and irradiation for recurrence are preferred therapies but often at the cost of significant treatment-related morbidity. Amputation provides excellent local control but may negatively affect patient quality of life without providing any impact on survival. Limb-salvage techniques using regional chemotherapy in the form of HILP or ILI are attractive alternatives to amputation and may be useful adjuncts to resection and irradiation. ILI, in particular, is well tolerated with favorable response rates, is repeatable and can be performed in the setting of distant disease. Patients with recurrent extremity STS should be referred for multidisciplinary consultation both for clinical trial consideration and to optimize treatment planning and potential for long-term outcome.
Abbreviations
CPK: Creatine Phosphokinase
HILP: Hypertheric Isolated Limb Perfusion
ILI: Isolated Limb Infusion
RT: Radiation Therapy
STS: Soft Tissue Sarcoma
References
- Cormier JN1, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54(2):94-109.
- Siegel RL, KD Miller, Jemal A. Cancer statistic, 2015. CA cancer J Clin. 2015. 65(1):5-29.
- Coindre JM1, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchère D, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914-26.
- Collin C, Hajdu SI, Godbold J, Friedrich C, Brennan MF. Localized operable soft tissue sarcoma of the upper extremity. Presentation, management, and factors affecting local recurrence in 108 patients. Ann Surg. 1987;205(4):331-9.
- Sim FH1, Pritchard DJ, Reiman HM, Edmonson JH, Schray MF. Soft-tissue sarcoma: Mayo Clinic experience. Semin Surg Oncol. 1988;4(1):38-44.
- Pisters PW1, Pollock RE. Staging and prognostic factors in soft tissue sarcoma. Semin Radiat Oncol. 1999;9(4):307-14.
- Davis AM1, Kandel RA, Wunder JS, Unger R, Meer J, O'Sullivan B, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66(2):81-7.
- Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003; 237(2):218-26.
- Trovik CS, Bauer HC, Alvegård TA, Anderson H, Blomqvist C, Berlin O, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36(6):710-6.
- Wilson AN, Davis A, Bell RS, O'Sullivan B, Catton C, Madadi F, et al. Local control of soft tissue sarcoma of the extremity: the experience of a multidisciplinary sarcoma group with definitive surgery and radiotherapy. Eur J Cancer. 30A(6):746-51.
- Singer S1, Corson JM, Gonin R, Labow B, Eberlein TJ. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994;219(2):165-73.
- Delaney TF, Kepka L, Goldberg SI, Hornicek FJ, Gebhardt MC, et al., Radiation therapy for control of soft-tissue sarcomas resected with positive margins. International journal of radiation oncology, biology, physics. 2007;67(5):1460-9.
- Sadoski C, Suit HD, Rosenberg A, Mankin H, Efird J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52(4):223-30.
- Eilber FC, Brennan MF, Riedel E, Alektiar KM, Antonescu CR, Singer S. Prognostic factors for survival in patients with locally recurrent extremity soft tissue sarcomas. Ann Surg Oncol. 2005;12(3):228-36.
- Moureau-Zabotto L, Thomas L, Bui BN, Chevreau C, Stockle E, Martel P, et al. Management of soft tissue sarcomas (STS) in first isolated local recurrence: a retrospective study of 83 cases. Radiother Oncol. 2004;73(3):313-9.
- Robinson M, Barr L, Fisher C, Fryatt I, Stotter A, Harmer C, et al. Treatment of extremity soft tissue sarcomas with surgery and radiotherapy. Radiother Oncol. 1990;18(3):221-33.
- Novais EN, Demiralp B, Alderete J, Larson MC, Rose PS, Sim FH. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res. 2010;468(11):3003-11.
- Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft tissue sarcomas of the lower extremity. Ann Surg. 1975;182(5):597-602.
- Williard WC, Collin C, Casper ES, Hajdu SI, Brennan MF. The changing role of amputation for soft tissue sarcoma of the extremity in adults. Surg Gynecol Obstet. 1992;175(5):389-96.
- Collin CF, Friedrich C, Godbold J, Hajdu S, Brennan MF. Prognostic factors for local recurrence and survival in patients with localized extremity soft-tissue sarcoma. Semin Surg Oncol. 1988;4(1):30-7.
- Collin C, Godbold J, Hajdu S, Brennan M. Localized extremity soft tissue sarcoma: an analysis of factors affecting survival. J Clin Oncol. 1987;5(4):601-12.
- Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996.14(5):1679-89.
- Creech O Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Annals of surgery, 1958;148(4):616-32.
- Hegazy MA, Kotb SZ, Sakr H, El Dosoky E, Amer T, Hegazi RA, et al. Preoperative isolated limb infusion of Doxorubicin and external irradiation for limb-threatening soft tissue sarcomas. Ann Surg Oncol. 2007;14(2):568-76.
- Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10(1):52-60.
- Grunhagen DJ, Brunstein F, Graveland WJ, van Geel AN, de Wilt JH, Eggermont AM. Isolated limb perfusion with tumor necrosis factor and melphalan prevents amputation in patients with multiple sarcomas in arm or leg. Ann Surg Oncol. 2005;12(6):473-9.
- Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BB, Schlag PM, Liénard D, et al., Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg. 1996;224(6):756-64.
- Gutman M, Inbar M, Lev-Shlush D, Abu-Abid S, Mozes M, Chaitchik S, et al., High dose tumor necrosis factor-alpha and melphalan administered via isolated limb perfusion for advanced limb soft tissue sarcoma results in a >90% response rate and limb preservation. Cancer. 1997;79(6):1129-37.
- Van Etten B, van Etten B, van Geel AN, de Wilt JHW, Eggermont AMM. Fifty tumor necrosis factor-based isolated limb perfusions for limb salvage in patients older than 75 years with limb-threatening soft tissue sarcomas and other extremity tumors. Ann Surg Oncol. 2003;10(1):32-7.
- Olieman AF, Pras E, van Ginkel RJ, Molenaar WM, Schraffordt Koops H, Hoekstra HJ. Feasibility and efficacy of external beam radiotherapy after hyperthermic isolated limb perfusion with TNF-alpha and melphalan for limb-saving treatment in locally advanced extremity soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 1998;40(4):807-14.
- Lejeune FJ Ghanem GE. A simple and accurate new method for cytostatics dosimetry in isolation perfusion of the limbs based on exchangeable blood volume determination. Cancer Res. 1987;47(2):639-43.
- Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14(3):238-47.
- Turaga KK, Beasley GM, Kane JM, Delman KA, Grobmyer SR, Gonzalez RJ, et al., Limb preservation with isolated limb infusion for locally advanced nonmelanoma cutaneous and soft-tissue malignant neoplasms. Arch Surg. 2011;146(7):870-5.
- Santillan AA1, Delman KA, Beasley GM, Mosca PJ, Hochwald SN, Grobmyer SR, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol. 2009;16(9):2570-8.
- Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18(10): 905-10.
- Vrouenraets BC, Klaase JM, Kroon BB, van Geel BN, Eggermont AM, Franklin HR. Long-term morbidity after regional isolated perfusion with melphalan for melanoma of the limbs. The influence of acute regional toxic reactions. Arch Surg.1995;130(1):43-7.
- Moncrieff MD1, Kroon HM, Kam PC, Stalley PD, Scolyer RA, Thompson JF. Isolated limb infusion for advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2008;15(10):2749-56.
- Vohra NA1, Turaga KK, Gonzalez RJ, Conley A, Reed D, Bui MM, et al. The use of isolated limb infusion in limb threatening extremity sarcomas. Int J Hyperthermia. 2013;29(1):1-7.