Case Report
Posterior Approach for Total Resection of a Sacral-Coccigeal Chordoma: A Case Report
Isabella Ponzio1, Del Bello L2, Monia Martiniani2, Leonard Meco2, Roberto Procaccini2, Nicola Giampaolini2 and Nicola Specchia2
1Orthopaedic Division, Republic of San Marino, Italy
2Department of Orthopaedics, University of Ancona, Italy
*Corresponding author: Monia Martiniani, Department of Orthopaedics, University of Ancona, via Conca 71, 60126 Ancona, Italy
Published: 02 Jan, 2018
Cite this article as: Ponzio I, Del Bello L, Martiniani M,
Meco L, Procaccini R, Giampaolini
N, et al. Posterior Approach for Total
Resection of a Sacral-Coccigeal
Chordoma: A Case Report. Clin Oncol.
2018; 3: 1395.
Abstract
A young patient refers progressive worst of lower back pain that became localized in the sacral area
getting worst while sitting or standing. On clinical examination, a small area over the coccyx gave
painful sensations on pressure, VAS scale was 7. Images from RMN showed a well defined coccygeal
mass that demonstrated a low signal on T2, bright signal on T1and a disomogeneal structure with
enhancement. Destruction of the coccyx without invasion of the gluteal musculature and rectum
were present and confirmed by Ct scan. The incisional biopsia was performed to make a certain
diagnosis of chordoma. Patient was undergone to radical excision from a posterior approach. The
lesion was excised en bloc with the coccyx and fourth to fifth sacral vertebra with at least 2 cm
healthy surgical margins. Combined use of post-operative high let radiation to increase diseasefree
interval was used. Follow–up was performed by MRI at 40 and 70 days after surgery showing
no chordoma recurrence. Effective management of coccygeal chordoma requires early diagnosis,
accurate preoperative staging, definitive and adequate surgical resection with proved tumor-free cut
margins and closed follow-up.
Keywords: Chordoma; Coccigeal; Sacral; Posterior; Resection
Introduction
Chordoma is a rare, low-grade, slow growing primary malignant tumor arising from primitive
nothocord remnants of the axial skeleton.
It accounts for 1-4 % of all primary skeletal tumors and its incidence rate is inferior to 0.1 per
100.000 inhabitants per year.
The most common location is the sacroccygeal region (40-50%) and the base of the skull (35-
40%), followed by the vertebral bodies (15-20%).
Chordoma biological behavior is characterized by a generally slow aggressive local growth with
a low tendency in metastatizing to distant sites including the lung, bone, soft tissue, lymph nodes,
liver, and skin. Although it is considered to be of low metastatic potential, up to 40-60% of patient
are, however, reporter to develop distant metastates over the course of their disease.
Adequate wide surgery still remains the cornerstore of chordoma treatment even though safe
margins are often hard to obtain because of its anatomical sites of origin.
The achievement of negative surgical margins favourably correlates with the rate of local relapse
and survival.
Sacrococcygeal chordomas are insidious tumors that are difficult to diagnose; many patients are
treated for an assortment of unrelated diagnoses before the correct diagnoses is made.
Case Report
The patient was a 39 years old Caucasian man who presented himself to his family physician
complaining in the last 6-7 years pain when sitting and especially when rising from the sitting
position [1-2].
Lower back pain was referred to multilevel lumbar erniated disc already knows to the patient.
From 2009 patient refers progressive worst of lower back pain that became localized in the lower
gluteal area and sacral area while sitting or after prolonged periods of standing.
Therefore he performs X-rays of the lumbosacral spine and
coccyx, and then the patient decides to contact us and arrived to our
observation [3].
On clinical examination, a small area over the coccyx gave painful
sensations on pressure, not detectable lump in this area could be
shown; on visual analogue pain scale (maximum=) -he defined his
pain between 7 and 8 before surgical intervention.
He was subsequently evaluated with magnetic resonance imaging
(Rmn) and computerized axial tomography (TC) , the images were
obtained in axial and sagittal planes using various T1 and T2 –
weighted sequences.
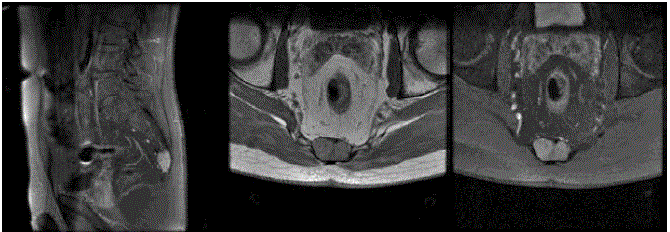
Images from RMN showed a large, well defined, lobulated mass
(measuring about 3x6x2 cm), identified at the coccix. Destruction of
the coccyx without insinuation into gluteal musculature and anterior
displacement of the rectum without invasion were present (Figure 1).
On MRI without enhancement the lesion demonstrated a low
signal on T2 -weighted images and bright signal on T1 waiting.
On RMI with enhancement the lesion demonstrated disomogeneal
structure.
Unfortunally TC scan was performed only in the axial plane [4].
The exam confirmed partial sacral (S5) and total coccygeal invasion;
in particular the images showed anterior and posterior coccygeal
cortical interrumptions.
Hematological and biochemical profiles were within normal
limits.
The results of images studies and clinical diagnostic procedures
were discussed in an interdisciplinary groundround composed by
orthopaedic and neurosurgeon.
The incisional biopsia was the first surgical procedure made to
gain a certain diagnosis [5].
Under antibiotic intravenous coverage of 2 gr cefazolina,
using a dorsal approach was performed incisional biopsia at level
of the terminal part of coccyx in the interglutealsolcus; a partial
skeletrization let us to expose distal portion of lesion.
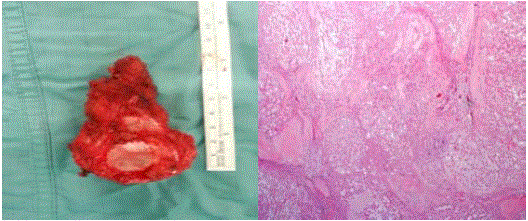
Macroscopically lesion has cerebroid aspect, soft texture, and
purple color (Figure 3).
Morfological and intraoperatory aspects suggest a diagnosis of
chordoma confirmed by istopathologic exams (Figure 3).
After three weeks the patient was undergone to radical excision.
The patient lies prone in Kraske’s position; skin median vertical
incision was about 20 cm long, from S2 to coccyx; the coccygeal
chordoma was excised en bloc with the coccyx and fourth to fifth
sacral vertebra [6]. The whole capsule of the tumor was kept intact and
at least 2 cm healthy surgical margins were achieved macroscopically.
The histopathological report confirmed free surgical margins [7]. The
patient recovered uneventfully and was discharged on the fifteenth
postoperative day. He was sent to the oncologist for the subsequent
treatment and follow-up.
Although chordoma is resistant to the effects of conventional
radiotherapy, our institution combined the use of post-operative high
let radiation with surgery to attempt to increase disease- free intervals
[8].
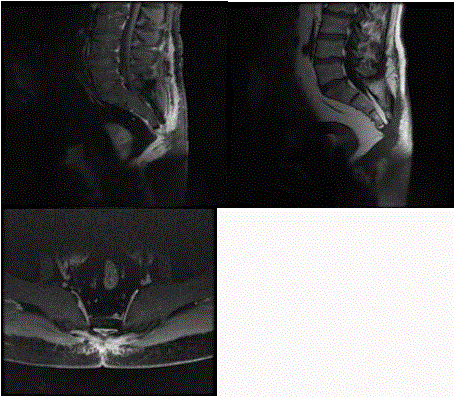
Follow –up was performed by MRI 40 days after surgery. It
showed fluid collection in the surgical site with irregular margins,
soft tissues enhancement. Probably these reports were compatible
with fibrous scar tissue development in the surgical area.
Next MRI made at 70 days after surgery displayed fluid collection
resolution with persistent soft tissue enhancement due to scar tissue
remodellation [9]. In the sub-coccigeal area, on the pelvic plane,
death tissue assumed nodular aspects (7, 3 x 4, and 5) with irregular
margins. These findings are not correlated to a chordoma recurrence
(Figure 2).
Figure 1
Figure 1
Destruction of the coccyx without insinuation into gluteal musculature and anterior displacement of the rectum without invasion were present.
Figure 2
Figure 3
Figure 3
Morfological and intraoperatory aspects suggest a diagnosis of
chordoma confirmed by istopathologic exams.
Conclusion
Chordoma account for less than 5 % of all bone tumours and it's
observed especially among 30 and 60 years with a predilection for
male sex (12). Due to its ectodermal origin, chordoma is not properly
a sarcoma even if it has clinically retained and classified as such being
a primary tumor of bone.
Its histological assessment is often delayed due to non typical
signs and symptoms of disease with a frequent clinical diagnosis of
pelvic or vertebral and irradiated pain due to discogenic or a specific
pathology.
Histology shows multiple lobules of abundant myxoid matrix
with cords, strands, and solid nests of phylaphorous cells, which show
enlarged atypical nuclei and eosinophilic cytoplasm with variable
sized vacuoles. Dedifferentiation and development of high grade
sarcoma like areas are possible.
Some of the sacrococcigeal region located pathologies are
chordoma, giant cell tumor, neurofriboma, teratoma, methastasis,
mixopapillaryepandimoma, myeloma, osteoblastoma, aneurysmal
bone cist, lipoma, osteosarcoma and condrosarcoma, anal fistulas
sacral dermatoids and microtraumas, post-partum lesions.
The slow modality of biologic growth, associated to a relatively
low incidence of metastic spread makes surgery the primary treatment
of this rare bone tumor [10-11].
The extension of margins is, in fact, a very important prognostic
factor being correlated with the incidence of local relapses and
overall survival. Local recurrence was significantly associated with an
increased risk of metastases and tumor-related deates.
Conventional radiotherapy with high energy photons is poorly
active and need , moreover , to be delivered in doses as high as 60-65
Gy, It may offer some temporary benefit in disease control in patients
with inadequate surgery( ie close or positive margins ) or , as exclusive
treatment , in patient with unresectable /inoperable disease.
Sensitivity to chemotherapy is very low and generally reported
in the small subgroup of patients with high grade dedifferentiated
chordomas with agents active in high grade sarcomas.
In the experience of Maggiore Hospital of Bologna (Italy), on 52
patients treated over a period of fifty years until 2002, only 20% of
patients operated on with safe margins had a local recurrence with a
relapse free survival of 56-94 months. This percentage has grown up
to 100% of patients who underwent radiotherapy alone or inadequate
surgery with intralesion margin with the detection of local recurrence
within 17-20 months from primary surgery.
Although potentially curative , a margin free “ en bloc” resection
is often very hard to obtain due to the anatomical sites of origin of
chordoma ie skull base , spine , and sacro-coccigeal area.
Considering the above situation, we conclude that effective
management of coccygeal chordoma require
s early diagnosis , accurate preoperative staging , definitive and
adequate surgical resection with proved tumor- free cut margins and
closed follow-up.
To conclude, despite the progress of current surgical techniques
and some encouraging results the with the use of targeted therapy ,
disease control and long term patients prognosis are still poor and
chordoma results generally , in a long –lasting life affecting disease.
Nevertheless, specific experience of the multidisciplinary team
(surgeons, medical oncologists, radiotherapists, pathologists,
radiologists) is a very important pre-requisite in succeeding to
improve patients’ quality of life and, hopefully, outcome.
References
- Ahmed AR. Safety margins inresection of sacral chordoma: analysis of 18 patients. Arch Orthop Trauma Surg. 2009;129(4):483-87.
- Apichat A and Saranatra W. Wide resection of Sacralchordoma via posterior approach. Int Orthop. 2012;36(3):607-12.
- Bergh P, Kindblom LG ,Gunteberg B, Remotti F, Ryd W and Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine : a study 39 patients. Cancer. 2000;88(9):2122-34.
- Casali PG, Stacchiotti S, Sangalli C, Olmi P and Gronchi A. Chordoma. Curr opin oncol. 2007;19(4):367-70.
- Cheng E, Ozerdemoglu R, Transfeldt E and Thompson R. Lumbosacral chordoma , prognostic factor and treatment. Spine (Phila Pa 1976). 1999;24(16):1639-16.
- Ferraresi, Nuzzo C, Zoccali C, Marandino F, Vidiri A, Salducca N, et al. Chordoma: clinical characteristics, management and prognosis of a case series of 25 patients. BMC Cancer. 2010;10:22.
- Gennari L , Azzarelli A and Quagliuolo V. A posterior approach for the excision of sacral chordoma. J Bone a Joint Surg Br. 1987;69(4):565-8.
- Genovese AM, Fedele F, et al.Minerva chirurgica. 2000;55:455-8.
- Haasper C, Langer F, Rosenthal H, Knobloch K, Mossinger E, Krettek C, et al. Coccydinya due to benign notochordalcelltumor. Spine (Phila Pa 1976). 2007;32(14):E394-6.
- Hakan S, Selcuk O, Handan D, Omur A and Tımurkaynak E. Total resection of inferiorly located sacral chordoma with posterior only approach: case report and review of the literature. Turk Neurosurg. 2010;20(4):527-32.
- Joseph H, Schwab, Healey J, Rose P, Casas-Ganem J and Boland P. The surgical management of sacral Chordomas. Spine (Phila Pa 1976). 2009;34(24):2700-04.