Research Article
Effects of Vialinins A and B on Murine Splenocytes Sensitized with Ovalbumin
Jun-ichi Onose, Fumiyo Sekiya, Aki Shiomitsu, Yasukiyo Yoshioka, Kouichi Sugaya and Naoki Abe*
Department of Nutritional Science and Food Safety, Faculty of Applied Bio-science, Tokyo University of Agriculture, Japan
*Corresponding author: Naoki Abe, Department of Nutritional Science and Food Safety, Faculty of Applied Bio-science, Tokyo University of Agriculture, Japan
Published: 10 Oct, 2017
Cite this article as: Jun-ichi Onose, Sekiya F, Shiomitsu
A, Yoshioka Y, Sugaya K, Abe N.
Effects of Vialinins A and B on Murine
Splenocytes Sensitized with Ovalbumin.
Clin Oncol. 2017; 2: 1357.
Abstract
Vialinins A and B, as strong inhibitors of the production and release of tumor necrosis factor (TNF)-α, have been isolated from the edible Chinese mush room, Thelephoravialis. Here we investigated the inhibitory effects of vialinins A and B on the immune system. Splenocytes obtained from ovalbumin (OVA)-sensitized BALB/c mice were challenged with OVA in the presence of vialinins A and B, and cytokine levels in the medium of cultured cells were measured. Vialinins A and B inhibited production of OVA-specific immunoglobulin (Ig)E and Th2-type cytokines (interleukin (IL)-4, IL-5, and IL-10) but not production ofTh1-type cytokines (interferon-γ, IL-2, and IL-12). Flow cytometric assay showed a significantly higher percentage of regulatory T cells (CD25- and Foxp3-positive T cells) among splenocytes cultured with OVA and vialinins A and B than among those cultured with OVA alone. This offers a first demonstration that vialinins A and B inhibit antigen-specific IgE- and Th2-type cytokines and regulation of regulatory T cells, suggesting the utility of vialinins A and B in preventing deleterious immune responses.
Introduction
Mushrooms have been used for centuries as folk medicines and food. The functionality of mush
rooms in disease prevention and medicinal treatment has been passed down through generations.
Many recent studies have shown significant agreement between the traditional uses of fungi in the
treatment of specific symptoms and experimental anti-bacterial, anti-fungal, anti-cancer, and antiviral
activities in laboratory trials [1]. Furthermore, medicinal mush rooms are reportedly effective
against inflammation [2]. Screening of medicinal mushrooms for bioactivity is thus extremely
important to identify sources of potential therapeutic agents. Vialinins A and B were isolated
from the dried fruiting bodies of Thelephoravialis (Thelephoraceae family), a Chinese mushroom
popular for its special flavor and taste. Moreover, this mushroom has long been used for the
treatment of low back pain and limb paralysis in China. Previous studies have revealed that vialin
in A displays a powerful 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging activity [3],
and inhibits the antigen-induced production and release of tumor necrosis factor (TNF)-α from
rat basophilic leukemia (RBL-2H3) cells (IC50:a half maximal inhibitory concentration = 0.09 nM)
and from murine bone marrow-derived mast cells (IC50 = 0.04 nM), compared with the clinical
immunosuppressant tacrolimus used in the same run as a positive standard (IC50= 0.25 nM) [4,5].
Vialinin B strongly inhibited TNF-α release induced by antigen from RBL-2H3 cells (IC50 = 0.02
nM) [6].On the other hand, analogs of vialinins A and B, comprisingganbajuninsB, D, and E,
atromentin, and cycloleucomelone, exhibited no inhibitory activity against TNF-α production in
RBL-2H3 cells [4,6].
Host immune responses are characterized by T-cell activation in response to antigen
stimulation, leading to the differentiation of effector T-cell subtypes that, in turn, are characterized
by distinct cytokine secretion and enzymatic profiles leading to specific effector functions. Efficient
host defense against invading pathogenic microorganisms is achieved through coordination of
complex signaling networks that link the innate and adaptive immune systems. Upon interaction
with cognate antigen presented by antigen-presenting cells such as dendritic cells (DCs), CD4+ T
cells can differentiate into a variety of effector subsets, Th1 cells, Th2 cells, Th17 cells, and induced
regulatory T cells(iTregs). The differentiation decision is governed predominantly by the cytokines
present in the microenvironment, and, to some extent, by the strength of the interaction of the
T-cell antigen receptor with antigen [7,8]. Th1 responses are induced by IL-12, composed of IL-
12α and IL-12β, triggering interferon (IFN)-γ production [9]. Th2 responses are characterized by
IL-4 and IL-13 production [10]. In addition, Th17 cells, characterized
by IL-17 production, cause recrudescence of autoimmune disease
[11]. Uncontrolled Th1 responses can lead to necrosis and tissue
damage, whereas exaggerated responses by Th2 cells can induce
asthma and allergy, and can also lead to tissue inflammation and
fibrosis [12]. Furthermore, transcription factors play important
roles in many immune disorders. Th1-cell differentiation requires
the action of T-box transcription factor (T-bet), Th2 differentiation
requires the action of GATA-binding protein 3 (GATA3), Th17-
cell differentiation requires retinoid-related orphan receptor (ROR)
γt, and Treg differentiation requires forkhead box P3 (Foxp3).
The present study examined the effects of vialinins A and B on the
production of specific immunoglobulin (Ig)E antibody in murine
splenocytes sensitized with ovalbumin (OVA).Furthermore, to
clarify the mechanisms underlying the inhibition of specific IgE
production, we examined the pattern of cytokine production by
vialinin-stimulated splenocytes from murine splenocytes sensitized
with OVA. In addition, we investigated the proportions of helper T
cells, Th1 cells, Th2 cells, Th17 cells, and Tregs in vialinin-stimulated
splenocytes from murine splenocytes sensitized with OVA.
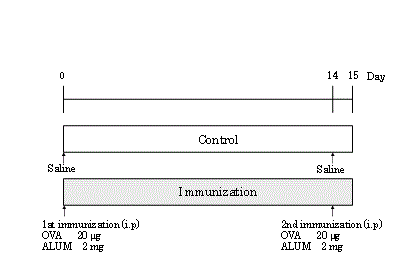
Figure 1
Figure 2
Figure 2
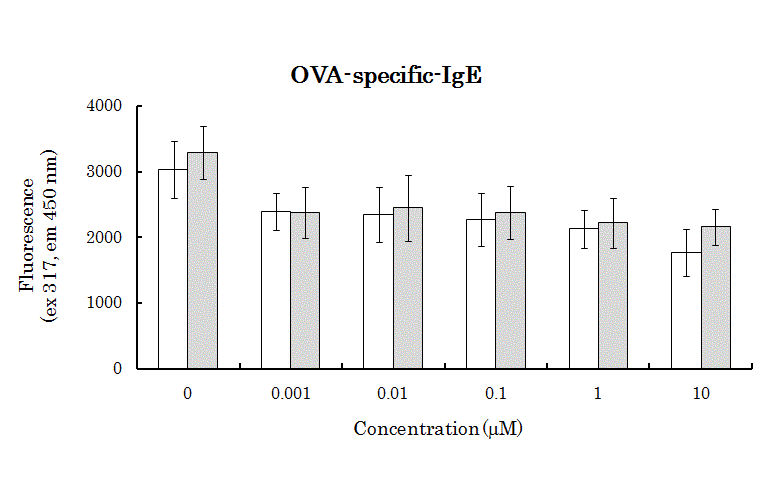
Effects of vialinins A and B on OVA-specific IgE production in
murine splenocytes. White bar,treated with vialinin A; grey bar,treated with
vialinin B. Data expressed as mean ± S.D. of five independent experiments.
Significance of differences from control values was estimated using Student’s
t-test (**p < 0.01, *p< 0.05).
Figure 3
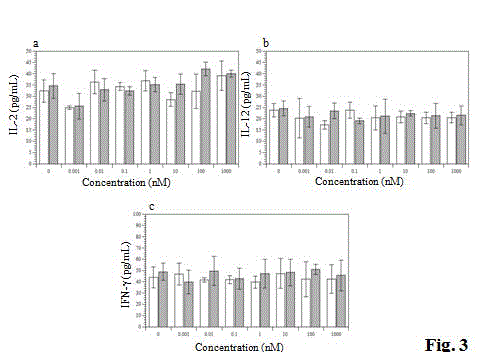
Figure 3
Effects of vialinins A and B on Th1-type cytokines production in
murine splenocytes. White bar, treated with vialinin A; grey bar, treated with
vialinin B. Data expressed as mean ± S.D. of five independent experiments.
Figure 4
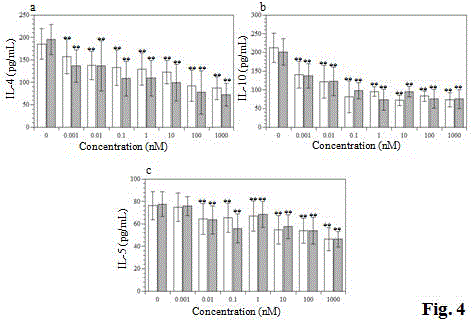
Figure 4
Effects of vialinins A and B on Th1-type cytokines production in
murine splenocytes. White bar, treated with vialinin A; grey bar, treated
withvialinin B. Data expressed as mean ± S.D. of five independent
experiments.Significance of differences from control values was estimated
using Student’s t-test (**p < 0.01).
Figure 5
Figure 5
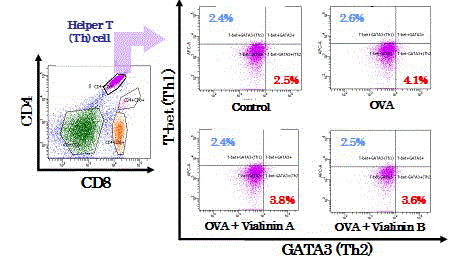
Expression of T-bet and GATA3 on murine splenocytes.
Splenocytes were isolated from the control, OVA treatment, OVA + vialinin
A and OVA + vialinin B groups. Histograms are representative of five
independent experiments.
Materials and Methods
Animals
Inbred specific-pathogen-free BALB/c mice (male, 6weeks old)
were purchased from Charles River Japan (Yokohama, Japan). Mice
were maintained in atemperature- and light-controlled environment
with free access to sterile diet and water, and were acclimatized for
at least 1 week before the start of the study.All experiments were
conducted according to the Guide for the Care and Use of Laboratory
Animals from theJapan Neuroscience Society and the Guide for the
Tokyo University of Agriculture.
Five mice were sensitized by intraperitoneal injection of 20 μg of
OVA and 2 mg of aluminum hydrate adjuvant (ALUM (Al(OH)3);
LSL, Shiga, Japan)in a total volume of 400 μl. Two weeks after the
first injection, they were given a booster injection of the same doses
of the antigens. The next day the mice were humanely sacrificed, and
their spleens were harvested. The immunization schedule is shown
in Figure 1.
Preparation and stimulation of murine splenocytes in vitro
Mice were sacrificed by cervical dislocation, and after removing
their spleens aseptically, cell suspensions were prepared by passing
the spleens through a sterile cells trainer (FALCON 35-2350). The cell
suspensions we rewashed twice in RPMI 1640 medium supplemented
with10% FBS, 2 mML-glutamine, 1 mM sodium pyruvate,50 U/ml
kanamycin sulfate, and 25 nM 2-ME, and they were adjusted to a
density of 5 ×106 cells/ml. Cells were plated at 2 ml/well in 24-well
cell culture clusters(Corning, NY) and challenged with OVA at a final
concentration of 100 μg/ml. Vailinins A or B (final concentration:1
μM), or saline was added to the 24-wellculture clusters, and it was
incubated at 37 °C in a CO2 incubator for 3-14 days. Vialinins A and
B were used as synthetic compounds [13,14]. Supernatants and cells
were then harvested to measure cytokine production and cell surface
markers.
OVA specific-IgE and cytokine production in vitro
The mouse serum OVA specific-IgE was determined by the
method. with some modifications. The cytokines examined in this
study were IFN-γ, IL-2, IL-4, IL-5, IL-10, and IL-12. The amounts
of these cytokines in the culture medium after incubation were
measured with ELISA kits (R&D Systems, Minneapolis, MN)based
on the quantitative sandwich enzyme immunoassay technique.
Absorbance was measured at 450nm using a precision micro plate
reader.
Flow cytometric analysis
Flow cytometric analysis was performed using a FACSAria flow
cytometer (BD Biosciences, San Jose, CA, USA)equipped with a 488
nm argon laser and detectors for forward scatter (FSC) and 90°light
scatter (side scatter, SSC) and for FL1 (band pass filter wavelength,
530 nm) and FL2 (585 nm) fluorescence emission in the green part
and red/orange part, respectively, of the spectrum. Splenocytes were
stained with phycoerythrin(PE)-labeled Cy7 Rat anti-mouse CD4,
allophycocyanin (APC)-labeled Cy7 Rat anti-mouse CD8a, Alexa
Fluor 488 Rat anti-mouse GATA3, PE-labeled Rat anti-mouse IL-
17a, APC-labeled Rat ant-mouse CD25, Alexa Fluor 488 Rat antimouse
Foxp3 (BD Biosciences), Alexa Fluor 647 Rat anti-mouse
T-bet (Santa Cruz Biotechnology, Dallas, Texas, USA), Anti-human/
mouse RORγt/RORC2/NR1F3-APC (R&D Systems). Fluorescence
overlap was compensated electronically by using splenocytes stained
with single colors, and then 10,000 cells were acquired and stored
for each analysis. Splenocytes were identified by their characteristic
appearance on a dot plot of FSC versus SSC and electronically gated
to exclude platelets, red cells, and dead-cell debris. The gate was the
same for splenocytes co-cultured with OVA and vialinin A, with
OVA and vialinin B, and with OVA alone. The results are reported
as percentages of positive cells within a gate. The absolute number of
each type of cell was calculated from the percentage of each type of
positive cell and the total number of splenocytes.
Statistical analysis
Numerical data are expressed as mean ± standard deviation of the
mean. Differences were evaluated using Student’s t-test, and values of
p< 0.01 were considered statistically significant.
Figure 6
Figure 6
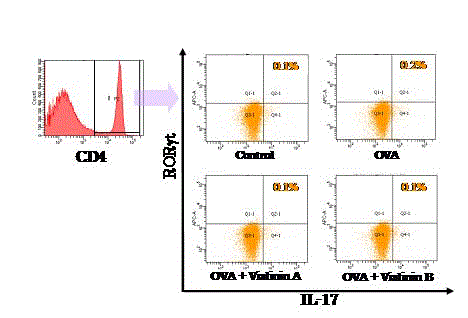
Expression of RORt and IL-17 on murine splenocytes. Splenocytes
were isolated from the control, OVA treatment, OVA + vialinin A and OVA
+ vialinin B groups. Histograms are representative of five independent
experiments.
Figure 7
Figure 7
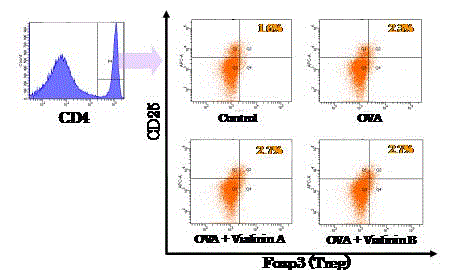
Expression of CD25 and Foxp3 on murine splenocytes.
Splenocytes were isolated from the control, OVA treatment, OVA + vialinin
A and OVA + vialinin B groups. Histograms are representative of five
independent experiments.
Results
Effects of vialinins A and B on production of OVA-specific
IgE from splenocytes
We investigated whether vialinins A and B suppress OVA-specific
IgE production. Although control splenocytes produced OVAspecific
IgE, splenocytes treated with vialinins A and B appeared
to suppress production of OVA-specific IgEin low concentration
(Figure. 2).
Effects of vialinins A and B on production of cytokine
from splenocytes
To clarify the inhibitory mechanisms of OVA-specific IgE
production in mice, we investigated production of Th1- and Th2-type
cytokines from murine splenocytes stimulated with OVA in vitro.
Cytokine release was expressed in the form of activity released into
the medium as a percentage of total activity. Vialinins A and B did not
influence Th1-type cytokine IL-12, IL-2 orIFN-γ (Figure.3). However
vialinins A and B also inhibited the Th2-type cytokines IL-4, IL-5, and
IL-10 in a dose-dependent manner (Figure.4).
Flow cytometricanalysis of splenocytes
To investigate the effects of vialinins A and B on differentiation
of murine splenocytes treated with OVA, we undertook phenotypic
analysis of murine splenocytes using FCM. Addition of vialinin A or
B to OVA-sensitive murine splenocytes decreased GATA3-positive
T cells,Th2 cells, but did not influence T-bet-positive T cells, Th1
cells (Figure 5).We also investigated the effects of vialinins A and
B on differentiation ofTh17 and Treg cells. Differentiation of Th17
cells, identified as RORγT-positive T cells, was not influenced by the
presence of vialinins A and B (Figure 6). However, Tregs, identified as
Foxp3-positive T cells, were increased among OVA-sensitive murine
splenocytes treated with vialinins A and B (Figure 7).
Discussion
We first demonstrated that the effects of vialinins A and B on
OVA-specific IgE production by urine splenocytes. Vialinins A
and B inhibited OVA-specific IgE production at concentrations of
0.001μM each (Figure 2). It has reported that oligodeoxynucleotide
from Bifidobacteriumlongum inhibited OVA-specific IgE production
by murine splenocytes [15]. Vialinins were not strongly inhibited as
compared with oligodeoxynucleotide but there were significantly
inhibited. Next, we investigated the effects of vialinins A and B on
Th1- and Th2-type cytokine production by murine splenocytes,
because IgE production is related to humoral immune response.
CD4+ helper T cells are subpopulations of two cell types, Th1 and
Th2, defined based on the different patterns of cytokine production
[16,17]. The balance of these two types of cells is considered to be
important for maintaining homeostasis in the host. Once this balance
becomes disturbed, various immunological diseases, such as allergies
and intestinal inflammation, can occur due to circumvention of
the host defense mechanisms. Regulation of these two types of cell
seems important for preserving host immune response, including IgE
and cytokine production. Vialinins A and B inhibited the Th2-type
cytokines IL-4, IL-5, and IL-10 from murine splenocytes treated with
OVA in a dose-dependent manner (Figure 4), but did not influence
theTh1-type cytokines IL-2, IL-12, and IFN-γ (Figure 3). We therefore
next investigated the effects of vialinins A and B on differentiation
of murine splenocytes, particularly helper T cells. Vialinins A and B
suppressed differentiation of GATA3-positive cells, representing Th2
cells, induced by OVA but showed no influence on the differentiation
of Th1 cells, as T-bet-positive cells (Figure 5). These findings suggest
that vialinins inhibit certain cytokines but do not influence the
balance of helper T cells (the Th1/Th2 paradigm). Recently, Th1/Th2/
Th17 and regulatory T-cell paradigm was spread into the mainstream
of mutually interacting immune network [18]. Vialinins A and B did
not influence differentiation of RORγt-positive Th17 cells (Figure 6),
but increased that of Tregs, as CD25- and Foxp3-positive T cells, in
murine splenocytes treated with OVA (Figure 7).
One of the deubiquitinating enzyme (DUB):ubiquitin-specific
protease 4 (USP4), reportedly promotes Th17 cell differentiation
in human naive T cells [19]. We have identified ubiquitin-specific
peptidase 5 (USP5), as a target molecule of vialinin A in RBL-2H3 cells,
and vialinin A inhibited USP5enzymatic activity in vitro. In addition,
vialinin A also strongly inhibited the activity of USP4 [20]. Though
TNF-α production was decreased in USP5 siRNA-knockdown RBL-
2H3 cells, no relationship was identified between USP4 and TNF-α
release [21]. The observation that vialinins as potent USP4 inhibitors
gave no differentiation activities of RORγt-positive Th17 in this OVA
treatment study, suggests that functions of DUBs may be different
depending on the sensitization level of T-cells.
Regulatory CD4+CD25+ T cells (Tregs) mediating immune
balance and immunopathologicalcontrol in the thymus are called
natural Tregs (nTregs) [12]. These nTregscan reach the periphery as
functional suppressor cells [22]. Tregs exert suppressive functions
either directly by producing IL-10 and TGF-β, or indirectly via
dendritic cells [23]. In some settings, a complex network of both
Foxp3+ Tregs and combinations of IL-10 and TGF-β has been found
to play an important role in controlling host immune responses.
However, reversal of these regulatory settings has been shown to
result in parasite clearance [24]. These findings suggest that vialinins
might control the immune balance via inhibition of Th2-type
cytokines and regulation of nTregsaccording to the Th1/Th2/Th17
and regulatory T-cell paradigm. Increasing Tregsis reportedly related
to small cell lung cancer and late-stage ovarian cancer [25], and
immune tolerance in the fetus and womb [26]. Tregs, as both nTregs
and iTregs, constitute an indispensable component of the immune
system. Further elucidation of the cellular functions of Tregs and the
molecular function of vialinins will contribute to our understanding
of immune tolerance and homeostasis and provide insights into
methods for achieving better control of immune responses for the
benefit of the host. Vialinins A and B are semi-specific DUB inhibitors
and therefore they have multiple points of action. The ubiquitin
pathway is necessary at all stages of development in eukaryotic
cells. Dynamic modification of a substrate protein by ubiquitin can
modify functions, localizations, and fate in the cell [27]. Ubiquitin
conjugation depends on a cascade of enzymes, and its removal is
mediated by DUBs. In recent years, attention has been focused on the
function of the ubiquitin-proteasome system, but many parts remain
unknown. Understanding the function of the ubiquitin-proteasome
system in immunology has contributed to the understanding of
immune-related diseases [28-30]. In conclusion, we demonstrated
that vialinins A and B inhibited Th2-type cytokines and antigenspecific
IgE production from murine splenocytes. Vialinins A and
B also controlled nTregs. Vialinins may be beneficial in preventing
various types of deleterious immune response.
References
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69-75.
- Jeong JW, Lee HH, Han MH, Kim GY, Hong SH, Park C, Choi YH. Ethanol extract of Poriacocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement Altern Med. 2014;14:101.
- Xie C, Koshino H, Esumi Y, Takahashi S, Yoshikawa K, Abe N, et al. a novel 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenger from an edible mushroom in China. BiosciBiotechnolBiochem.2005;69:2326.
- Onose J, Xie C, Ye Y, SugayaQ, TakahashiK, KoshinoS, et al. Pharm Bull. 2008;31:831.
- Onose J, Yoshioka Y, Ye Y, SugayaQ, YajimaK, TaniguchiA, et al. Cell Immunol. 2012;279:140.
- Xie C, Koshino H, Esumi Y, Onose J, Yoshikawa K, Abe N. Vialinin B, a novel potent inhibitor of TNF-alpha production, isolated from an edible mushroom, Thelephoravialis. Bioorg Med Chem Lett. 2006;16(20):5424-6.
- Boyton RJ, Altmann DM. Is selection for TCR affinity a factor in cytokine polarization? Trends Immunol. 2002;23(11):526-9.
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646-55.
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune-cell function in mice with disrupted interferon-γ genes. Science. 1993;259(5102):1739-42.
- Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10(2):137-42.
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-40.
- Dewals B, Hoving JC, Horsnell WG, Brombacher F. Control of Schistosomamansoni egg-induced inflammation by IL-4-responsive CD4+ CD25-CD103+ Foxp3-cells is IL-10-dependent. Eur J Immunol. 2010;40(10):2837-47.
- QY Yue, H Koshino, J Onose ,K Yoshikawa , N Abe , S Takahashi. First total synthesis of vialinin A, a novel and extremely potent inhibitor of TNF-alpha production.Org Lett. 2007;9(21):4131-4.
- Ye YQ, Koshino H, Onose J, Yoshikawa K, Abe N, Takahashi S. Expeditious synthesis of vialinin B, an extremely potent inhibitor of TNF-alpha production.Org Lett. 2009;11(21):5074-7.
- Takahashi N, Kitazawa H, Iwabuchi N, Xiao JZ, Miyaji K, Iwatsuki K, Saito T. Immunostimulatory oligodeoxynucleotide from Bifidobacteriumlongum suppresses Th2 immune responses in a murine model.Clin ExpImmunol. 2006;145(1):130-8.
- Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. AdvImmunol. 1989;46:111-47.
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;175:5-14.
- S Saito, A Nakashima, T Shima, M Ito. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am J ReprodImmunol. 2010;63(6):601-610.
- Yang J, Xu P, Han L, Guo Z, Wang X, Chen Z, et al. Ubiquitin-specific protease 4 promotes Th17 cell function under inflammation by deubiquitinating and stabilizing RORγt. J Immunol. 2015;194(9):4094-7.
- Okada K, Yue YQ, Taniguchi K, Yoshida A, Tomonori A. Vialinin A is a ubiquitin-specific peptidase inhibitor. Bioorg Med Chem Lett.2013;05:093.
- Yoshioka Y, Ye YQ, Okada K, Taniguchi K, Yoshida A, Sugaya K, et al. Ubiquitin-specific peptidase 5, a target molecule of vialinin A, is a key molecule of TNF-α production in RBL-2H3 cells. PLoS One. 2013;8(12):e80931.
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor- chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151-64.
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu Rev Immunol. 2009,27:551-89.
- Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinellaspiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039-47.
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766-72.
- Rowe JH, Ertelt JM, XinL. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102-6.
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159-80.
- Misaghi S, Balsara ZR, Catic A, Spooner E, Ploegh HL. Chlamydia trachomatis-derived deubiquitinating enzymes in mammalian cells during infection. MolMicrobiol.2006;61:142-50.
- Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol Cell. 2005;19:547-57.
- Angot A, Vergunst A, Genin S, PeetersN. Exploitation of eukaryotic ubiquitin signaling pathways by effectors translocated by bacterial type III and type IV secretion systems. PLoSPathog.2007;3:e3.