Case Report
Urinary Bladder Cancer - Old Models, New Opportunities
Oliveira PA*, Vala H, Gil da Costa RM, Pinto-Leite MR, Arantes-Rodrigues R and Vasconcelos-Nóbrega C
Department of Cytogenetic Laboratory, Hospital Center of Trás-os-Montes and Alto Douro, Portugal
*Corresponding author: Oliveira PA, Department of Cytogenetic Laboratory, Hospital Center of Trás-os- Montes and Alto Douro, Portugal
Published: 14 Jun, 2017
Cite this article as: Oliveira PA, Vala H, Gil da Costa RM,
Pinto-Leite MR, Arantes-Rodrigues
R, Vasconcelos-Nóbrega C. Urinary
Bladder Cancer - Old Models, New
Opportunities. Clin Oncol. 2017; 2:
1349.
Abstract
Urinary bladder cancer remains as one of most frequent tumours. To elucidate the reasons for the
development of tumours, to find out which factors determine the tumour progression and to develop
new and better treatments with fewer side effects, intensive research, with the combination of in
vitro and in vivo studies is mandatory. So, in this manuscript we performed a revision concerning
the different methodologies to study this disease. In authors’ opinion, the best strategy to improve
scientific knowledge for UBC should always rely in the association of in vivo and in vitro results.
Currently, there are still many challenges in UBC diagnosis and therapy. These challenges must be
faced as new opportunities. The molecular diagnostics and genomic revolution will be fundamental
to develop new therapeutic modalities, and also to promote personalized therapies.
Keywords: Rat; Mouse; Carcinogenesis
Introduction
Urinary Bladder Cancer (UBC) remains as one of most frequent tumours, being the second
most frequent tumour of the genitourinary tract, only surpassed by the prostate cancer [1-3]. UBC
ranges from mild disease, with a low mortality rate (however with high recurrence), to extremely
high-grade tumors, associated with metastasis and high mortality. Notably, UBC is one of the most
costly cancers to treat, primarily due to the considerable costs associated with life-long clinical
management of patients with non-muscle invasive disease, as well as those associated with the cost
of caring for patients after surgical removal of the urinary bladder [4-6]. With a clear correlation
with environmental exposures, such as tobacco smoke, industrial chemicals, dietary nitrates and
arsenic [1,7-12] UBC affects more men than women (2-3x).
Nevertheless, despite its prevalence and adverse impact on human health, UBC has been
remarkably understudied relative to other cancers [6].
To elucidate the reasons for the development of tumours, to find out which factors determine
the tumour progression and to develop new and better treatments with fewer side effects, intensive
research, with the combination of in vitro and in vivo studies, is mandatory.
In Vitro Studies: Cell Lines
Cancer cell lines are routinely used for various kinds of biomedical research, from drugsensitivity
tests to identify potential therapy targets and pharmacologically useful compounds [13-
15]. For the study of UBC, several cell lines have been established. T24, HT1376, 5637, UM-UC-3 are
some examples of human bladder cancer cell lines [13]. Some have origin in superficial tumours but
the majority are from invasive and metastatic ones [13,14]. Cell lines from experimental UBC are
fewer than those of human origin. AY-27 and NBT-II are UBC cell lines derived from rats [13,16-
18]. BTT-T739 and MB49 are examples of bladder cancer cell lines with origin in mice [13,19].
In vitro studies with cell lines demand a great amount of care concerning the origin of cell lines.
It is crucial to ensure that they are reliable, because cell cross-contamination is a common problem
during cell culturing and use. Cross-contamination provides misleading research results leading to
unusable therapeutic products. The unwitting use of misidentified cell lines may, ultimately, expose
patients to inappropriate, or even harmful, treatments.
In urological research, cell lines, particularly human urothelial cell lines, are well-established
tools for preclinical trials. For most cytotoxic agents, if it does not work in vitro, it will most certainly
not work in vivo. If it works in vitro, then there is the possibility it may be effective in vivo. They are
a cost-efficient method of searching for drug activity and can further our understanding of drugs’
action on several tumours. Other advantages in using cell lines can also be highlighted: they are easy
to handle and can be replicated almost infinitely. Additionally, they
exhibit a relatively high degree of homogeneity. Cell lines, however,
have some disadvantages. They are prone to genotypic and phenotypic
drift during their continual culture. Subpopulations may arise and
cause phenotypic changes over time by the selection of specific, more
rapidly growing clones within a population [20].
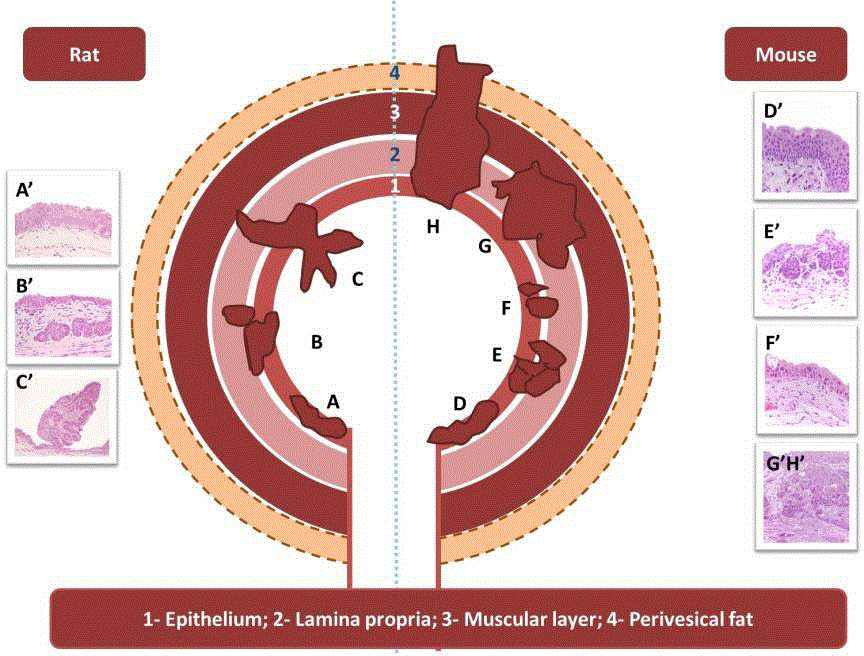
Figure 1
Figure 1
Scheme of the invasion status of bladder tumors in rat and mouse models. Rat – Non-invasive tumours. Lesions are located primarily at the epithelium and eventually lamina propria. [A and A’: Simple hyperplasia; A’: (H&E, 200x); B and B’: Nodular hyperplasia; B’: (H&E, 200x);C and C’: Papilloma; C’: (H&E,
100x)]; Mouse: Invasive tumors that can penetrate deeper layers of the bladder such as muscular layer, perivesical tissue and extravesical organs. [D and D’:
Simple hyperplasia; D’: (H&E, 600x); E and E’: Nodular hyperplasia; E’: (H&E, 400x); F and F’: Carcinoma in situ; F’: (H&E, 400x); G, G’, Hand H’: Invasive
carcinoma; G’, H’: (H&E, 200x)].
In Vivo Studies: Animal Models
There is a dire need for relevant animal models for research to
improve the treatment and management of humans (and animals)
with UBC.
Animal models have greatly contributed to the understanding of
carcinogenesis, establishing the bridge between in vitro laboratory
investigations and studies in humans [21]. Animal models allow
the investigation of aspects that cannot be studied under clinical
conditions, such as the evaluation of new chemotherapeutic,
immunotherapeutic or prophylactic agents, drug regimens, or other
treatment methods and can also provide further information on basic
mechanisms of tumour growth and spread [22,23].
Although there is the possibility to use several animal species,
rodents (rats and mice) are those most often used in animal
experimentation [24-38]. UBC can be established subcutaneously
(heterotopically) by transplantation of tumour cells, or intravesically
(orthotopically) either by transplantation of tumour cells or by
chemical induction [19,22,30].
Our team has developed an intense research work with chemically
induced bladder cancer models, namely rats and mice [39-49]. They
are orthotopic models, developed in immunocompetent animals.
These models have the great advantage of simulating the local cancer
environment where the influence of the immune system and the
anatomical and physiological factors of the tissue of origin, which
undoubtedly influence the metastatic process, are not affected
[19,30,32,39].
Rodent Models of UBC Chemically Induced
The rodent models of chemically induced UBC can be used to test
prophylactic drugs, to test therapeutic drugs and also to define the
impact of chemical carcinogens on other organs. There are several
chemical carcinogens that can be used to induce bladder cancer in
experimental animals. N-butyl-N-(4-hydroxybutyl) nitrosamine
(BBN), N-[4-(5-nitro-2furyl)-2-thiazolyl] formamide (FANFT) and
N-methyl-N-nitrosourea (MNU), are some examples [33-34,50-
52], being the first one (BBN) the most used carcinogen in UBC
experimental oncology studies.
BBN is an indirect carcinogen: after ingestion it is metabolized
mainly in the liver, but also in the urinary bladder, into several
metabolites that reach the urinary bladder through urine and come
into contact with the urothelium, binding covalently to cellular
macromolecules and initiating the carcinogenic process [53,54-57].
BBN-induced urothelial lesions in rodents resemble human
urothelial lesions in their morphological characteristics. However,
the spectrum of urothelial lesions is different in rats and in mice.
Rat model of UBC resembles non-invasive bladder cancer of
humans, with papillary neoplasm, while mouse model resembles
flat urothelial lesions evolving to invasive bladder cancer. In rat
model of UBC, it is possible to observe simple hyperplasia, dysplasia,
nodular hyperplasia, papilloma and papillary neoplasm. In mouse
model, urothelial lesions that arise from the BBN administration are
different. They can be initiated with simple hyperplasia, progress to
displasia, carcinoma in situ and later on invasive carcinoma.
Figure 1, summarizes the development of urothelial lesions in rats
and mice, also showing the invasion grade of the lesions.
Both rodent models of chemically induced bladder cancer are
extremely useful in urologic oncology research, since they represent
two variables of the same disease in humans. If the non-invasive
pathway has a better prognosis, it is also true that it can relapse. Invasive
pathway, studied in mice models, similarly with the development of
UBC in humans, can metastasize. It is important to understand these
differences in order to delineate better experiments, according to its
main purpose and to achieve improved and translatable results. If the
idea of the use of these models is not new, the study possibilities that
they offer are never ending.
In authors’ opinion, the best strategy to improve scientific
knowledge for UBC should always rely in the association of in vivo
and in vitro results. Currently, there are still many challenges in
UBC diagnosis and therapy. These challenges must be faced as new
opportunities. The molecular diagnostics and genomic revolution
will be fundamental to develop new therapeutic modalities, and also
to promote personalized therapies.
Acknowledgments
This work is financed by national funds through FCT -
Fundação para a Ciência e Tecnologia, I.P., under the project
UID/Multi/04016/2016. Furthermore we would like to thank the
InstitutoPolitécnico de Viseu and CI&DETS for their support.
This work is supported by European Investment Funds by
FEDER/COMPETE/POCI– Operacional Competitiveness and
Internacionalization Programme, under Project POCI-01-0145-
FEDER-006958 and National Funds by FCT - Portuguese Foundation
for Science and Technology, under the project UID/AGR/04033/2013.
Furthermore we would like to thank to UTAD and CITAB for their
support.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67(1): 7-30.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61(2): 69-90.
- Winer E, Gralow J, Diller L, Karlan B, Loehrer P, Pierce L, et al. Clinical Cancer Advances 2008: Major research advances in cancer treatment, prevention, and screening-a report from the American Society of Clinical Oncology. J Clin Oncol. 2009; 27(5): 812-826.
- Botteman MF, Pashos CL, Hauser RS, Laskin BL, Redaelli A. Quality of life aspects of bladder cancer: a review of the literature. Qual Life Res. 2003; 12(6): 675-688.
- Yeung C, Dinh T, Lee J. The Health Economics of Bladder Cancer: An Updated Review of the Published Literature. Pharmacoeconomics. 2014; 32(11): 1093-1104.
- Kobayashi T, Owczarek TB, McKiernan JM, Abate-Shen C. Modelling bladder cancer in mice: opportunities and challenges. Nat Rev Cancer. 2015; 15(1): 42-54.
- de Braud F, Maffezzini M, Vitale V, Bruzzi P, Gatta G, Hendry WF, et al. Bladder cancer. Crit Rev Oncol Hematol. 2002; 41(1): 89-106.
- Fadl-Elmula I. Chromosomal changes in uroepithelial carcinomas. Cell Chromosome. 2005; 4: 1.
- Volanis D, Kadiyskaa T, Galanisb A, Delakasa D, Logothetic S, Zoumpourlisc V. Environmental factors and genetic susceptibility promote urinary bladder cancer. Toxicol Lett. 2010; 193(2): 131-137.
- Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of some aromatic amines, organic dyes, and related exposures. Lancet Oncol. 2008; 9(4): 322-323.
- Clapp RW, Jacobs MM, Loechler EL. Environmental and occupational causes of cancer: new evidence 2005-2007. Rev Environ Health. 2008; 23(1): 1-37.
- Takkouche B, Regueira-Méndez C, Montes-Martínez A. Risk of cancer among hairdressers and related workers: a meta-analysis. Int J Epidemiol. 2009; 38(6): 1512-1531.
- Gabriel U, Bolenz C, Michel MS. Experimental Models for Therapeutic Studies of Transitional Cell Carcinoma. Anticancer Res. 2007; 27(5A): 3163-3171.
- Hatina J, Huckenbeck W, Rieder H, Seifert HH, Schulz WA. [Bladder carcinoma cell lines as models of the pathobiology of bladder cancer. Review of the literature and establishment of a new progression series]. Urologe A. 2008; 47(6): 724-34.
- Tsuji K, Kawauchi S, Saito S, Furuya T, Ikemoto K, Nakao N, et al. Breast cancer cell lines carry cell line-specific genomic alterations that are distinct from aberrations in breast cancer tissues: Comparison of the CGH profiles between cancer cell lines and primary cancer tissues. BMC Cancer. 2010; 10: 10-15.
- Nishi M. A cell line derived from BBN (N-butyl-N-[4-hydroxybutyl]-nitrosamine)-induced rat bladder cancer: establishment and scanning electron microscopic cell surface characteristics. Acta Med Okayama. 1978; 32(3): 181-205.
- Chen JJ, Ye ZQ, Koo MW. Growth inhibition and cell cycle arrest effects of epigallocatechin gallate in the NBT-II bladder tumour cell line. BJU Int. 2004; 93(7): 1082-1086.
- Hojo H, Kaneko A, Kayagaki N, Saki M, Hashimoto Y. Subcellular localization and characterization of interleukin-1 alpha produced by rat bladder cancer cells. Immunol Lett. 1994; 43(3): 215-220.
- Arentsen HC, Hendricksen K, Oosterwijk E, Witjes JA. Experimental rat bladder urothelial cell carcinoma models. World J Urol. 2009; 27(3): 313-317.
- Burdall SE, Hanby AM, Lansdown MR, Speirs V. Breast cancer cell lines: friend or foe? Breast Cancer Res. 2003; 5(2): 89-95.
- Reis LO, Pereira TC, Favaro WJ, Cagnon VHA, Lopes-Cendes I, Ferreira U. Experimental animal model and RNA interference: a promising association for bladder cancer research. World Journal of Urology. World J Urol. 2009; 27(3): 353-361.
- Günther JH, Jurczok A, Wulf T, Brandau S, Deinert I, Jocham D, et al. Optimizing syngeneic orthotopic murine bladder cancer (MB49). Cancer Res. 1999; 59(12): 2834-2837.
- Steele VE, Lubet RA. The use of animal models for cancer chemoprevention drug development. Semin Oncol. 2010; 37(4): 327-338.
- Okajima E, Hiramatsu T, Hirao K, Ijuin M, Hirao Y, Babaya K, et al. Urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in dogs. Cancer Res. 1981; 41(5): 1958-1966.
- Cohen AE, Weisburger EK, Weisburger JH, Ward JM, Putnam CL. Cystoscopy of chemically induced bladder neoplasms in rabbits administered the carcinogen dibutylnitrosamine. Invest Urol. 1975; 12(4): 262-266.
- Cohen SM. Cell proliferation and carcinogenesis. Drug Metab Rev. 1998; 30(2): 339-357.
- Hueper WC. Aniline tumors of the bladder. Arch Pathol. Invest Urol. 1975; 12(4): 262-266.
- Kunze E, Chowaniec J. Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of the urinary bladder. IARC Sci Publ. 1990; (99): 345-397.
- Clayson DB, Fishbein L, Cohen SM. Effects of stones and other physical factors on the induction of rodent bladder cancer. Food Chem Toxicol. 1995; 33(9): 771-784.
- Chan ES, Patel AR, Smith AK, Klein JB, Thomas AA, Heston WD, et al. Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. J Urol. 2009182(6): 2926-2931.
- Chan E, Patel A, Heston W, Larchian W. Mouse orthotopic models for bladder cancer research. BJU Int. 2009; 104(9): 1286-1291.
- Levett D, Flecknell PA, Rudland PS, Barraclough R, Neal DE, Mellon JK, et al. Transfection of S100A4 produces metastatic variants of an orthotopic model of bladder cancer. Am J Pathol. 2002; 160(2): 693-700.
- Lijinsky W. Chemistry and biology of N-nitroso compounds. In: Cambridge Monographs on Cancer Research. Cambridge University Press, Cambridge UK. 1992; 67-82.
- druckrey H, Preussmann R, Ivankovic S, Schmidt Ch, Mennel Hd, Stahl Kw. [Selective Induction Of Bladder Cancer In Rats By Dibutyl- And N-Butyl-N-Butanol(4)-Nitrosamine]. Z Krebsforsch. 1964; 66: 280-290.
- Swaminathan S, Bryan GT. Biotransformation of the bladder carcinogen N-[4-(5-nitro-2-furyl)-2-thiazolyl] formamide in mice. Cancer Res. 1984; 44(6): 2331-2338.
- Cui L, Shi Y, Daib G, Panb H, Chen J, Song L, et al. Modification of N-methyl-N-nitrosourea initiated bladder carcinogenesis in Wistar rats by terephthalic acid. Toxicol Appl Pharmacol. 2006; 210(1-2): 24-31.
- Reis LO, Fávaro WJ, Ferreira U, Billis A, Fazuoli MG, Cagnon VH. Evolution on experimental animal model for upper urothelium carcinogenesis. World J Urol. 201028(4): 499-505.
- Martín-Sanz P, Mayoral R, Casado M, Boscá L. COX-2 in liver, from regeneration to hepatocarcinogenesis: what we have learned from animal models? World J Gastroenterol. 2010; 16(12): 1430-1435.
- Oliveira PA, Pires MJ, Nóbrega C, Arantes-Rodrigues R, Calado AM, Carrola J, et al. Technical report: Technique of bladder catheterization in female mice and rats for intravesical instillation in models of bladder cancer. Scand J Lab Anim Sci. 2010;37(4):253-259.
- Vasconcelos-Nóbrega C, Colaço A, Lopes C, Oliveira PA. Review: BBN as an urothelial carcinogen. In Vivo. 2012; 26(4): 727-739.
- Oliveira PA, Vasconcelos-Nóbrega C, Gil da Costa R, Arantes-Rodrigues R. The N-butyl-N-4-hydroxybutyl Nitrosamine Mouse Urinary Bladder Cancer Model. Methods in Molecular Biology. Methods Mol Biol. 2018; 1655: 155-167.
- Oliveira PA, Gil da Costa RM, Vasconcelos-Nóbrega C. Challenges with in vitro and in vivo experimental models of urinary bladder cancer for novel drug discovery. Expert Opin Drug Discov. 2016; 11(6): 599-607.
- Oliveira PA, Arantes-Rodrigues R, Vasconcelos-Nóbrega C. Animal models of urinary bladder cancer and their application to novel drug discovery. Expert Opin Drug Discov. 2014; 9(5): 485-503.
- Alvarado A, Arantes-Rodrigues R, Vasconcelos-Nóbrega C, Da Costa R, Pinto-Leite MR, Faustino-Rocha AI. Urinary bladder chemical carcinogenesis in laboratory rodents as an experimental model [carcinogénesis química de vejiga urinaria en roedores de laboratorio como modelo experimental]. Revista Venezolana de Oncologia. 2015; 27: 57-63.
- Pinto-Leite R, Carreira I, Melo J, Ferreira SI, Ribeiro, Ferreira J, et al. Genomic characterization of three urinary bladder cancer cell lines: Understanding genomic types of urinary bladder cancer. Tumor Biology. Tumour Biol. 2014; 35(5): 4599-4617.
- Vasconcelos-Nóbrega C, Pinto-Leite R, Arantes-Rodrigues R, Ferreira R, Brochado P, Cardoso ML, et al. In vivo and in vitro effects of RAD001 on bladder cancer. Urologic Oncology: Seminars and Original Investigations. 2013; 31: 1212-1221.
- Arantes-Rodrigues R, Colaço A, Pinto-Leite R, Oliveira PA. In Vitro and In Vivoexperimental models as tools to investigate the efficacy of antineoplastic drugs on urinary bladder cancer. Anticancer Res. 2013; 33(4): 1273-96.
- Palmeira C, Oliveira PA, Lameiras C, Amaro T, Silva VM, Lopes C, et al. Biological similarities between murine chemical-induced and natural human bladder carcinogenesis. Oncol Lett. 2010; 1(2): 373-377.
- OliveiraPA, ColacoA, De la CruzP LF, Lopes C. Experimental bladdercarcinogenesis-rodent models. Exp Oncol. 2006; 28(1): 2-11.
- Swaminathan S, Bryan GT. Biotransformation of the bladder carcinogen N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide in mice. Cancer Res. 1984; 44(6): 2331-2338.
- Cui L, Shi Y, Dai G, Pan H, Chen J, Song L, et al. Modification of N-Methyl-N-Nitrosourea initiated bladder carcinogenesis in Wistar rats by terephthalic acid. Toxicol Appl Pharmacol. 2006; 210(1-2): 24-31.
- Reis LO, Fávaro WJ, Ferreira U, Billis A, Fazuoli MG, Cagnon VH. Evolution on experimental animal model for upper urothelium carcinogenesis. World J Urol. 2010; 28(4): 499-505.
- Cohen SM. Urinary bladder carcinogenesis. Toxicol Pathol. 1998; 26(1): 121-127.
- Iida K, Itoh K, Maher JM, Kumagai Y, Oyasu R, Mori Y, et al. Nrf2 and p53 cooperatively protect against BBN-induced urinary bladder carcinogenesis. Carcinogenesis. 2007; 28(11): 2398-2403.
- Suzuki E, Okada M. Metabolic fate of N-butyl-N-(4-hydroxybutyl)nitrosamine in the rat. Gan. 1980; 71(6): 856-862.
- Mochizuki M, Suzuki E, Okada M. [Structure and metabolic fate of N-nitrosodialkylamines in relation to their organotropic carcinogenicity with special reference to induction of urinary bladder tumors]. Yakugaku Zasshi. 1997; 117(10-11): 884-894.
- Cohen SM, Ohnishi T, Clark NM, He J, Arnold LL. Investigations of rodent urinary bladder carcinogens: collection, processing, and evaluation of urine and bladders. Toxicol Pathol. 2007; 35(3): 337-347.