Short Communication
Photo-Enhanced Delivery of Genetic Material Using Curcumin Loaded Composite Nanocarriers
Shashank Reddy Pinnapireddy#, Lili Duse#, Dena Akbari and Udo Bakowsky*
Department of Pharmaceutics and Biopharmaceutics, University of Marburg, Germany
#Both authors contributed equally to this work
*Corresponding author: Udo Bakowsky, Department of Pharmaceutics and Biopharmaceutics, University of Marburg, Robert-Koch-Str. 4, Marburg-35037, Germany
Published: 03 Aug, 2017
Cite this article as: Pinnapireddy SR, Duse L, Akbari D,
Bakowsky U. Photo-Enhanced Delivery
of Genetic Material Using Curcumin
Loaded Composite Nanocarriers. Clin
Oncol. 2017; 2: 1323.
Keywords
Photodynamic therapy; Photochemical internalisation; Gene therapy; Liposomes; Polyethylenimine; Lipopolyplexes
Introduction
Since several ages, light has been used as a therapeutic agent for the treatment of psoriasis,
vitiligo, rickets, and skin cancer [1]. Photo Dynamic Therapy (PDT) is the therapeutic utilisation
of light with or without the use of an adjuvant. An otherwise inter photosensitiser delivered to the
target tissue is activated using light at a specific wavelength. Upon photo-activation, in presence
of molecular oxygen, reactive oxygen species are generated which selectively destroy the tumour
tissue [2,3]. PDT has already been approved by the Food and Drug Administration (FDA) and
European Medicines Agency (EMA) for the treatment of certain pre-malignant and malignant
diseases [4,5]. Curcumin, a naturally occurring multifaceted drug, obtained from the rhizomes of
Curcuma longa has been used extensively for its antibacterial and antitumor properties and more
recently for photodynamic therapy [6]. A subcategory of photodynamic therapy, Photo Chemical
Internalisation (PCI) is the use of light for the breakdown of endo/lysosomal membranes to facilitate
release of their respective contents. This process is driven by the presence of photosensitisers in
these membranes which upon photo-activation initiate photochemical reactions causing rupture
of the vesicles leading to the release of the endocytosed contents [7,8]. PCI could be used for both
triggered release and enhanced release of therapeutically active substances and biomolecules [9,10].
Recent advances in LED technology have enabled development of flexible, portable and costeffective
light sources for photodynamic therapy. A prototype LED powered irradiating device,
capable of irradiating at different wavelengths was used in this study. To solve the solubility
problems of curcumin, we have used liposomes for encapsulating the curcumin. Liposomal
encapsulation increases the bioavailability, biocompatibility and bio efficacy of the therapeutic
substances ranging from drugs to biomolecules such as DNA, siRNA and other oligonucleotides
[11-13]. Poly Ethylen Imine (PEI) on the other hand is a proven polymer for gene delivery and is
often considered as a gold standard for transfection [14-16]. A combination of PEI/nucleic acid
polyplexes and liposomes called Lipopolyplexes (LPP) has been proven to increase the efficiency
of gene delivery and decrease the toxicity associated with polyethylenimine [17-19]. In this study,
we present the use of a novel combination of curcumin loaded liposomes and PEI/DNA polyplexes
for photochemical internalisation. Keeping in mind the toxicological aspect of PEI, whose
toxicity is related to the chemical structure and molecular weight, a linear and highly deacylated
variant of PEI, lPEI with a molecular weight of 22kDa was used in this study [20,21]. The cationic
polyplexes were encapsulated inside anionic liposomes formulated using 1,2-Dioleoyl-sn-glycero-
3-phosphoethanolamine (DOPE), Dipalmitoylphosphatidylcholine (DPPC), cholesterol and
curcumin. Since the transfection efficiency is highly dependent on the surface charge, size, flexibility
and stability of the liposomes, a helper lipid DOPE was incorporated in the liposomal formulation
which influences the above parameters [22-24].
Ovarian carcinoma is one of the most common cancer types, ranking fifth in the number
of deaths caused (WHO estimate). With a risk rate 1 in 75 women getting ovarian cancer
and a mortality rate of 1 in 100, conjugative tumour therapeutics involving gene therapy and
photodynamic therapy offer a promising adjunct to the prevailing therapies. We demonstrate
the use of Curcumin Loaded Lipopolyplexes (cLPP) for photochemical internalisation in
SK-OV-3 human adenocarcinoma cells (Figure 1). Cytotoxicity of the complexes has been
evaluated using MTT assay. Haemolysis assay using fresh blood
and activated partial thromboplastin time test using plasma were
used to demonstrate the biocompatibility of the curcumin loaded
lipopolyplexes. Physicochemical and structural analysis was done
using dynamic light scattering, laser Doppler velocimetry and
Transmission Electron Microscopy (TEM) respectively.
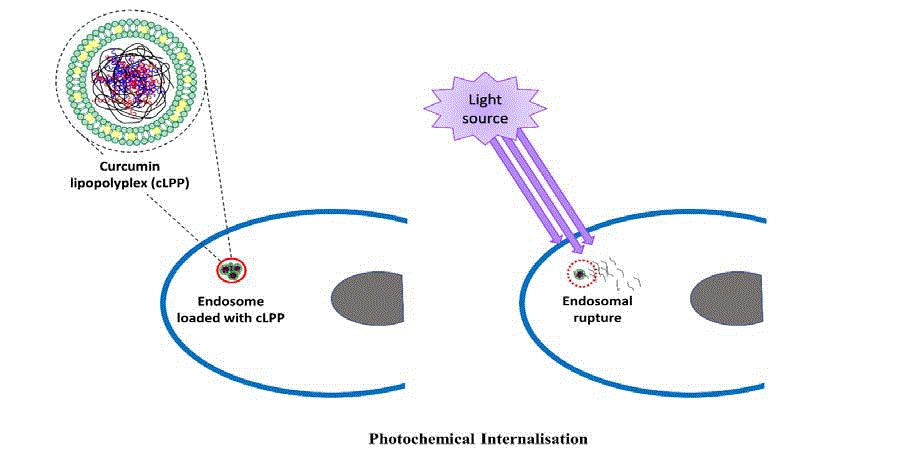
Figure 1
Figure 1
Scheme of photochemical internalisation showing endosomal rupture following photo-activation of curcumin lipopolyplexes leading to release of the
endosomal contents into the cytosol. Inset showing an enlarged illustration of curcumin lipopolyplex with curcumin entrapped between the liposomal bilayer and a
polyplex encapsulated within the liposome.
Materials and Methods
Liposomes were prepared using thin film hydration method as
described previously [25]. Briefly, chloroform, methanolic mixtures
of lipids (DOPE, DPPC and cholesterol; molar ratios 70, 15, 15
respectively) were added to a round bottomed flask and evaporated
using a rotary evaporator equipped with a vacuum pump (Laborota
4000, Heidolph Instruments, Schwabach, Germany) to obtain a thin
lipid film. In case of curcumin containing liposomes, curcumin was
dissolved in methanol was added to the lipid mixture at a ratio of 1:300
(curcumin: total lipid). The lipid film was rehydrated using 20 mM
HEPES buffer (pH 7.4) and sonicated in a bath sonicator to facilitate
liposome formation. The liposomes were extruded through 400 nm
and 200 nm polycarbonate membranes to obtain small unilamellar
liposomes. A fully deacylated 22kDa variant of linear PEI containing
11% more protonable nitrogens was used for polyplex formation
with luciferase expressing pCMV-luc plasmid. Lipopolyplexes were
prepared as previously described [26]. Briefly, both polyplexes and
liposomes (mass ratio 1:0.5) were mixed vigorously and incubated
for 1h at room temperature to facilitate lipopolyplexes formation.
Dynamic Light Scattering (DLS) and laser Doppler velocimetry
(using Zeta Sizer Nano ZS; Malvern Instruments Ltd., Malvern, UK)
were used for determining the hydrodynamic diameter and zeta
potential of the lipopolyplexes respectively. For transmission electron
microscopy, the lipopolyplexes containing curcumin were diluted
(1:10) using 10 mM HEPES buffer (pH 7.4) and mounted onto 300
mesh formvar coated copper grids. The samples were negatively
stained using 2% uranyl acetate and examined under an accelerating
voltage of 300 kV, with a current density of 55pA/cm2 using a JEM
3010 UHR transmission electron microscope (JEOL Ltd, Tokyo,
Japan). Images were acquired using a high-res slow scan CCD camera
(Gatan Inc., Pleasanton, USA).
For transfection experiments, SK-OV-3 cells seeded in a 96-
well plate (seeding density 10,000 cells/well) were incubated with
lipopolyplexes (with and without curcumin) containing 0.2 μg
pDNA/well mixed with IMDM culture medium containing 10%
foetal bovine serum. Lipopolyplexes without curcumin and untreated
cells were used as controls. 4h after incubation with lipopolyplexes,
the cells were irradiated at a wavelength of 457 nm with a radiation
fluence of 0.5 J/cm2, 1 J/cm2 and 3.2 J/cm2 using a prototype LED
irradiation device (Generation I LED irradiator; Lumundus GmbH,
Eisenach, Germany) custom manufactured to fit microtiter plates.
Post-irradiation, the cells were incubated at 37°C and 7.5% CO2 under
humid conditions for 48h. The cells were lysed using cell culture
lysis buffer (Promega GmbH, Mannheim, Germany) and analysed
using luciferase assay and Pierce protein assay to evaluate luciferase
expression. Luciferase release was used as function transfection
efficiency and was determined by measuring the luminescence using
luminometer (BMG Labtech, Offenburg, Germany) followed by the
addition of luciferase assay reagent mixture.
Similar steps were followed for the cytotoxicity assay with the
exception that after 48h MTT dye (3-(4,5-dimethylthiazol-2-yl)-2,5-
diphenyltetrazolium bromide) was added to cells and incubated for
further 4h. Subsequently the medium was aspirated and the formazan
crystals were dissolved using dimethyl sulphoxide. The absorbance
was read at 570 nm in a plate reader. Haemolysis was performed
using erythrocytes isolated from fresh blood obtained after prior
consent. The erythrocytes were diluted 1:50 with isotonic NaCl
(0.9%) and mixed 1:1 with lipopolyplexes containing 2μg pDNA.
As controls, 1% Triton™ X-100, isotonic NaCl and blood were used.
Haemolysis caused by Triton™ X-100 were considered as 100%. For
activated partial thromboplastin time test, plasma separated from
fresh blood was mixed with lipopolyplexes containing 2 μg pDNA
and evaluated using an TEClot aPTT-S kit (TECO GmbH, Neufahrn,
Germany) according to the manufacturer’s protocol in a Coatron M1
coagulation analyser (TECO GmbH). Isotonic NaCl and plasma were
used as controls.
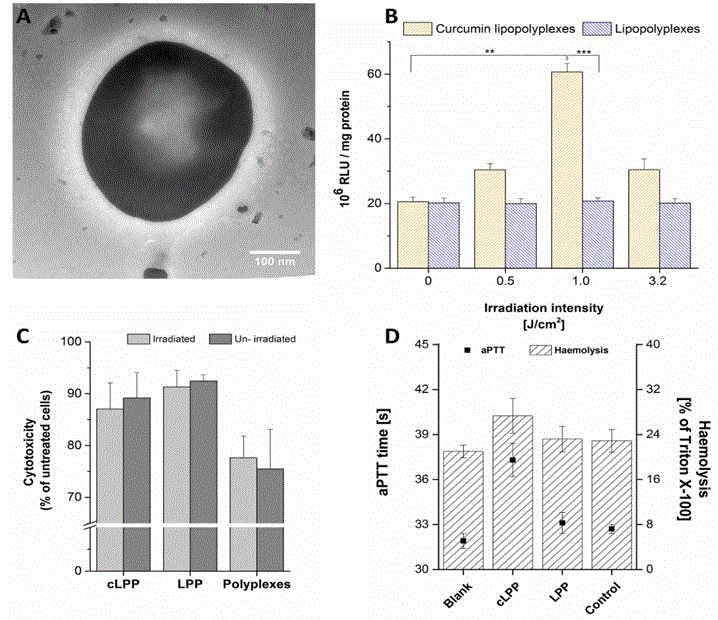
Figure 2
Figure 2
A.) Transmission electron micrograph of a curcumin lipopolyplex; scale bar 100 nm. B.) Photochemical internalisation in SK-OV-3 cells with curcumin
lipopolyplexes and lipopolyplexes without curcumin as a control. Irradiation intensity of 0 indicates dark i.e. un-irradiated cells. Values are presented as the mean
± SD (n = 3) and statistical differences (two-tailed Student’s t-test) are denoted as "**" p <0.01, "***" p <0.001. C.) MTT cytotoxicity assay of lipopolyplexes with
and without curcumin in SK-OV-3 cells at an irradiation intensity of 3.2 J/cm2. D.) Activated partial thromboplastin time test (in seconds; left y-axis) and haemolysis
assay (as % of haemolysis caused by Triton™ X-100; right y-axis) of lipopolyplexes with and without curcumin. Blank indicates plasma in case of aPTT test and
blood in case of haemolysis assay, control represents isotonic NaCl.
Results and Discussion
The structure of the cLPP could be visualised using TEM show
a spherical structure with a size of ~ 200 nm (Figure 2A) which
was within the size range for efficient cellular uptake [27]. The size
of the cLPP was consistent with that of the size obtained by DLS
measurements (195 nm ± 6.2 nm). The zeta potential of the cLPP
performed using laser Doppler velocimetry was found to be +8.6 mV
± 1.7mV. Taking into consideration the time required for endocytosis
to occur (1h - 4h), irradiation was performed at time intervals of 1h,
2h and 4h [28]. Substantial results could only be obtained after an
incubation time of 4h, therefore, subsequent irradiation experiments
were carried out after 4h. Evaluation of the PCI experiments showed
a dramatic improvement in the luciferase expression of irradiated
SK-OV-3 cells incubated with cLPP (Figure 2B). This effect was
pronounced at an irradiation fluence of 1 J/cm2 which is seen as an
optimal dosage in the experiments. However, at higher irradiation
doses, this effect diminishes pointing towards photo bleaching of
the curcumin, there by making it unavailable for further energy
level transitions which is a key for photochemical reactions [29]. The
absence of a similar effect in case of lipopolyplexes without curcumin
confirms that the PCI was dependent upon the photosensitiser
curcumin present inside the lipopolyplexes. Similarly, no
improvement in the transfection efficiency was seen in un-irradiated
(dark) experiments, pointing towards an activation of curcumin only
on the presence of LED light at 457 nm. The known mechanism of
action of photochemical internalisation is the energy level transition
during which generation of singlet oxygen occurs. This singlet oxygen
is responsible for the oxidising different cellular organelles [30]. Due
to its short lifetime and short range of action, only cellular targets
close to the photosensitiser are oxidised by the generation of singlet
oxygen [31].
No considerable increase in cytotoxicity was observed between
irradiated and dark cells incubated with LPPs with or without curcumin
(Figure 2C). This might be due to the limited range and limited
lifetime of the singlet oxygen generated during the photochemical
reactions [29]. To get a deeper insight into the biocompatibility of
our delivery system, we have performed haemocompatibility studies.
Haemocompatibility studies also serve as a crucial link between
in vitro and in vivo studies, the data whereof could be utilised to
customise the dosage in an in vivo setup. Haemolysis assay gives the
measure of damage to the erythrocytes, by measuring the amount
of haemoglobin released. Only a slight increase in the haemolytic
potential was observed in case of cLPP compared to LPP (Figure 2D).
Similar was the case for aPTT test wherein only a slight increase (5s)
in coagulation time could be noticed (Figure 2C). The coagulation
time of the plasma tested was found to be 32s ± 0.1s, values between
30s - 40s are considered normal, with values above 50s having clinical
significance. It is worth mentioning that for the haemocompatibility
tests, 10x concentration of the PCI experiments was considered. The
haemolysis effect observed with lipopolyplexes is very low and taking
into consideration, the ratio (v/v) of erythrocytes to injected delivery
vehicles in the human body, is negligible. A PTT results suggest that
the cLPPs and did not interfere with the coagulation pathway.
Conclusion
The multicomponent system in this study makes use of the proven lipopolyplexes in addition with curcumin for enhancing gene delivery via photochemical internalisation using a novel LED irradiation device. The use of curcumin loaded liposomes along with PEI/DNA polyplexes brings in the beneficial properties of the two systems together resolving issues related to bioavailability, biocompatibility and transfection efficiency. The PCI experiments in SK-OV-3 cells have shown at least a threefold increase in the transfection efficiency. The delivery system and the irradiation dose required for PCI were relatively non-toxic to the cells. Haemocompatibility studies have revealed the system to biocompatible, thereby making way for its use in vivo. Previous PCI studies for gene delivery used co-delivery of photosensitisers and gene delivery vehicles or by chemically linking the photosensitiser to the delivery vehicle. In our study, however, we have presented a system capable of introducing both the photosensitiser and genetic material together. This multi-component system has the potential to bring together photodynamic therapy and gene therapy opening new doors in the field of combination therapies for cancer treatment. Optimising this system for use against therapeutic targets in vitro and in vivo in chorioallantoic membrane model would be of our prime research focus in future.
Acknowledgement
The authors would like to thank Mrs. Eva M. Mohr for her support and technical assistance. The authors would also like to express gratitude towards Lumundus GmbH for designing the prototype LED irradiation device.
Contribution
Shashank Reddy Pinnapireddy: Optimisation of transfection
conditions, haemocompatibility studies, electron microscopy.
Lili Duse: Optimisation of irradiation parameters, cytotoxicity
studies, manuscript drafting.
Dena Akbari: Lipopolyplex preparation, photo-enhancement
studies.
References
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003; 3(5): 380-387.
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998; 90(12): 889-905.
- Stewart F, Baas P, Star W. What does photodynamic therapy have to offer radiation oncologists (or their cancer patients)? Radiother Oncol. 1998; 48: 233-248.
- Lou PJ, Jäger HR, Jones L, Theodossy T, Bown SG, Hopper C. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br J Cancer. 2004; 91(3): 441-446.
- Pais-Silva C, de Melo-Diogo D, Correia IJ. IR780-loaded TPGS-TOS micelles for breast cancer photodynamic therapy. Eur J Pharm Biopharm. 2017; 113: 108-117.
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013; 15(1): 195-218.
- Berg K, Folini M, Prasmickaite L, Selbo PK, Bonsted A, Engesaeter BØ, et al. Photochemical internalization: a new tool for drug delivery. Curr Pharm Biotechnol. 2007; 8(6): 362-372.
- Berg K, Weyergang A, Prasmickaite L, Bonsted A, Høgset A, Strand MT, et al. Photochemical internalization (PCI): a technology for drug delivery. Methods Mol Biol. 2010; 635: 133-145.
- Shieh MJ, Peng CL, Lou PJ, Chiu CH, Tsai TY, Hsu CY, et al. Non-toxic phototriggered gene transfection by PAMAM-porphyrin conjugates. J Control Release. 2008; 129(3): 200-206.
- Puri A. Phototriggerable Liposomes: Current Research and Future Perspectives. Pharmaceutics. 2014; 6(1): 1–25.
- Engelhardt KH, Pinnapireddy SR, Baghdan E, Jedelska J, Bakowsky U. Transfection Studies with Colloidal Systems Containing Highly Purified Bipolar Tetraether Lipids from Sulfolobus acidocaldarius, Archaea. 2017 (2017).
- Mahmoud G, Jedelská J, Strehlow B, Bakowsky U. Bipolar tetraether lipids derived from thermoacidophilic archaeon Sulfolobus acidocaldarius for membrane stabilization of chlorin e6 based liposomes for photodynamic therapy. Eur J Pharm Biopharm. 2015; 95(Pt A): 88-98.
- Janich C, Pinnapireddy SR, Erdmann F, Groth T, Langner A, Bakowsky U, et al. Fast therapeutic DNA internalization - A high potential transfection system based on a peptide mimicking cationic lipid. Eur J Pharm Biopharm. 2017;118: 38-47.
- Aigner A. Delivery Systems for the Direct Application of siRNAs to Induce RNA Interference (RNAi) In Vivo. J Biomed Biotechnol. 2006; 2006: 71659.
- Erbacher P, Bettinger T, Brion E, Coll JL, Plank C, Behr JP, et al. Genuine DNA/polyethylenimine (PEI) complexes improve transfection properties and cell survival. J Drug Target. 2004; 12(4): 223-236.
- Möhwald M, Pinnapireddy SR, Wonnenberg B, Pourasghar M, Jurisic M, Jung A, et al. Aspherical, Nanostructured Microparticles for Targeted Gene Delivery to Alveolar Macrophages. Adv Healthc Mater. 2017.
- Ewe A, Schaper A, Barnert S, Schubert R, Temme A, Bakowsky U, et al. Storage stability of optimal liposome-polyethylenimine complexes (lipopolyplexes) for DNA or siRNA delivery. Acta Biomater. 2014;10(6): 2663-2673.
- Ewe A, Panchal O, Pinnapireddy SR, Bakowsky U, Przybylski S, Temme A, et al. Liposome-polyethylenimine complexes (DPPC-PEI lipopolyplexes) for therapeutic siRNA delivery in vivo. Nanomedicine. 2017; 13(1): 209-218.
- Schäfer J, Höbel S, Bakowsky U, Aigner A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials. 2010; 31(26): 6892-6900.
- Kafil V, Omidi Y. Cytotoxic Impacts of Linear and Branched Polyethylenimine Nanostructures in A431 Cells. Bioimpacts. 2011; 1(1): 23-30.
- Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005; 102(16): 5679-5684.
- Kneuer C, Ehrhardt C, Bakowsky H, Kumar MN, Oberle V, Lehr CM, et al. The influence of physicochemical parameters on the efficacy of non-viral DNA transfection complexes: a comparative study. J Nanosci Nanotechnol. 2006; 6(9-10): 2776-2782.
- Zuhorn IS, Oberle V, Visser WH, Engberts JB, Bakowsky U, Polushkin E, et al. Phase behavior of cationic amphiphiles and their mixtures with helper lipid influences lipoplex shape, DNA translocation, and transfection efficiency. Biophys J. 2002; 83(4): 2096-2108.
- Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, et al. Nonbilayer phase of lipoplex–membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther. 2005; 11(5): 801-810.
- Brüssler J, Marxer E, Becker A, Schubert R, Schümmelfeder J, Nimsky C, et al. Correlation of structure and echogenicity of nanoscaled ultrasound contrast agents in vitro. Colloids Surf B Biointerfaces. 2014; 117: 206-215.
- Pinnapireddy SR, Duse L, Strehlow B, Schäfer J, Bakowsky U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf B Biointerfaces. 2017; 158: 93-101.
- Kumar MNVR, Bakowsky U, Lehr CM. Nanoparticles as Non-Viral Transfection Agents, in: Nanobiotechnology. Wiley-VCH Verlag GmbH & Co. KGaA. 2005; 319-342.
- Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014; 9 Suppl 1: 51-63.
- Høgset A, Prasmickaite L, Selbo PK, Hellum M, Engesaeter BØ, Bonsted A, et al. Photochemical internalisation in drug and gene delivery. Adv Drug Deliv Rev. 2004; 56(1): 95-115.
- Ohtsuki T, Miki S, Kobayashi S, Haraguchi T, Nakata E, Hirakawa K, et al. The molecular mechanism of photochemical internalization of cell penetrating peptide-cargo-photosensitizer conjugates. Sci Rep. 2015; 5: 18577.
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991; 53(4): 549-553.