Research Article
Association of Oral Contraceptives and Hormone Replacement Therapy with Pancreatic Cancer Risk in Women: A Meta-Analysis
Jin-Xiao Liang1, Wei Gao2, Xin-ming Zhou1, Yong Liang3* and Jun Ling4*
1Department of Thoracic Surgery, Zhejiang Cancer Hospital, Republic of China
2Department of Biochemistry and Molecular Biology, Dalian Medical University, Republic of China
3Department of Clinical Medicine, Taizhou University Medical School, Republic of China
4Department of Basic Sciences, Geisinger Commonwealth School of Medicine, USA
*Corresponding author: Jun Ling, Department of Basic Sciences, Geisinger Commonwealth School of Medicine, Scranton, PA 18509, USA
Published: 26 Jun, 2017
Cite this article as: Liang J-X, Gao W, Zhou X-M,
Liang Y, Ling J. Association of
Oral Contraceptives and Hormone
Replacement Therapy with Pancreatic
Cancer Risk in Women: A Meta-
Analysis. Clin Oncol. 2017; 2: 1309.
Abstract
Although the incidence rate of pancreatic cancer is higher in men than in women, Oral Contraceptives
(OC) and Hormone Replacement Therapy (HRT) commonly used in women may constitute a risk
or protective factor for pancreatic cancer. Some studies have examined these relationships, but
no coherent conclusions have been reached. Thus, we conducted a meta-analysis in this study by
extensive search of literature using PubMed and EMBASE databases. It was found that OC use was
associated with the significant decrease in pancreatic cancer risk (pooled OR 0.75, 95% CI 0.61-0.91).
Subgroup analysis identified that OC decreased the risk in the pancreatic cancer population-based
studies (pooled OR 0.76, 95 % CI 0.67-0.86) but was controversial in the hospital-based studies
(pooled OR 1.21, 95% CI 0.94-1.57). HRT did not show the significant effect on pancreatic cancer
risk (pooled OR 1.00, 95 % CI 0.79-1.26). However, subgroup analysis identified the decreased risk
in North America population (pooled OR 0.81, 95% CI 0.65-1.01) but the increased risk in Europe
population (pooled OR 1.51, 95% CI 1.16-1.97). In conclusion, OC use was demonstrated to have
a protective effect against pancreatic cancer in women, where as HRT showed no effect or diverse
effects in different populations. Our results increase the awareness of using HRT in women when
considering pancreatic cancer risk. The subgroup analysis also suggests that more clinical studies
are needed to clarify the specific relationship between OC or HRT and pancreatic cancer risk in
different populations.
Keywords: Pancreatic cancer; Oral contraceptives; Hormone replacement therapy; Risk factor,
Meta-analysis
Introduction
Pancreatic Cancer (PCA) is a high malignant carcinoma and the fourth leading cause of
cancer-related death in both men and women in the United States [1]; the incidence rate is higher
in men than in women [2]. Because of its insidious onset, the diagnosis of pancreatic cancer is
usually delayed, leading to the 5-year survival rate at less than 5% in the US and Europe. Identified
risk factors include cigarette smoking, overweight and obesity, diabetes, chronic pancreatitis and
family history of pancreatic cancer [3]. However, the reproductive factors have not drawn enough
attention, although there are some studies on the steroid hormone signaling in PCA [4,5]. By the
fact of lower PCA risk in women, female sex hormones, especially estrogen, may play a protective
role in PCA development and progression. Since contraceptives (OC) and Hormone Replacement
Therapy (HRT) are common practice in women world-wide, which perturb the endogenous balance
of steroid hormones, it is important to confirm the relationship between the use of OC and HRT
and PCA risk in women. Epidemiologic studies so far have generated controversial results, thus it is
imperative to perform a meta-analysis to help clarify this issue.
Exogenous use of hormones like OC and HRT has been known to play certain roles in cancer
development. OC containing estrogen and progestin has been identified to associate with reduced
risks of ovarian and endometrial cancers but with increased risks of cervical, liver and breast
cancers [6]. HRT is also commonly used in women to relieve adverse symptoms in pre- and postmenopausal
periods. With its similar components as OC, it is also known to correlate with lower
risks of uterine cervix, liver and biliary tract cancers but with higher
risks of endometrium, breast, and malignant melanoma cancers [7].
Compared to these types of cancer, the effects of OC and HRT on
women’s pancreatic cancer have not generated consistent results [8-
19], which support a rationale for us to carry out a systematic review
and meta-analysis in this study.
Figure 1
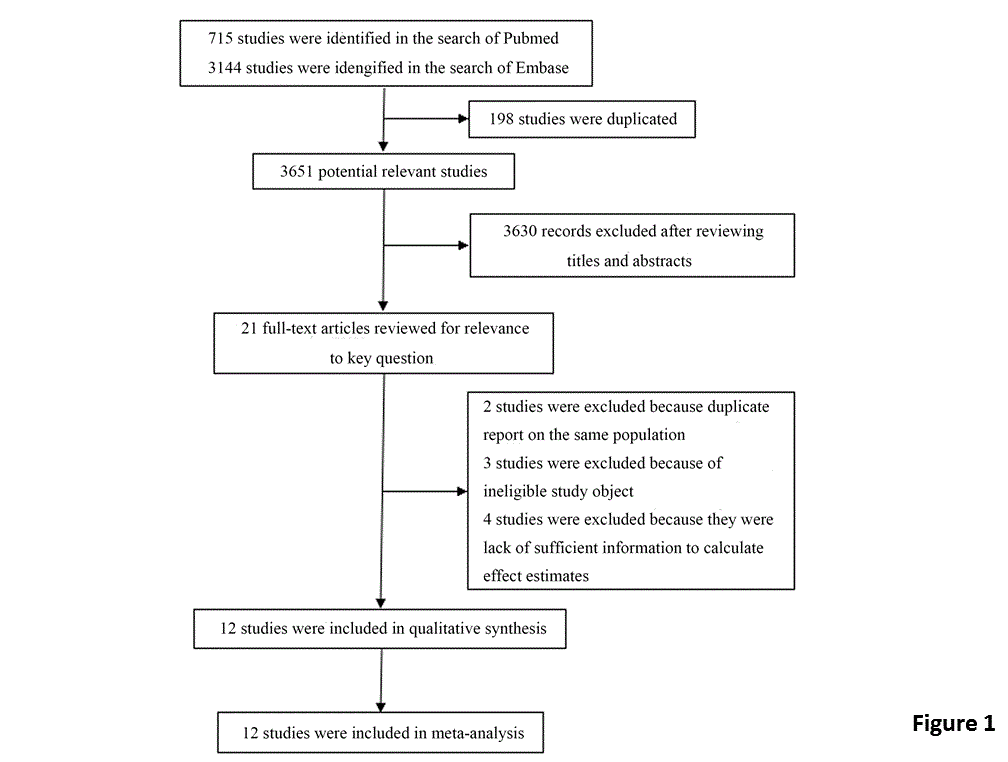
Figure 1
The process of literature search, screening and analysis to select studies to be included in this meta-analysis.
Methods
Literature search
Studies published from January 1900 to April 2014 were
identified by conducting literature searches in PubMed and EMBASE
databases. The search was limited to publications in English by using
the following search terms: (estrogens or estrogen replacement
therapy or hormone replacement therapy or gonadal hormones or
gonadal steroid hormones or sex hormones or oral contraceptives
or contraceptive or postmenopausal or reproductive factors) and
(pancreas or pancreatic) and (cancer or neoplasms or carcinoma
or tumor or adenocarcinoma). Furthermore, the reference lists of
research reports and review articles were also examined to identify
additional related publications. The study was conducted and
reported in accordance with the PRISMA (Preferred Reporting Items
for Systematic Reviews and Meta-Analyses) guidelines.
Study selection
To select the studies that were eligible for the meta-analysis, the
following criteria were used:
a) Published in English
b) Used a case-control or cohort study design
c) Reported the association between OC or HRT and pancreatic
cancer risk
d) Presented sufficient data for the calculation of the odds ratio
(or) and 95% confidence interval (95% CI).
Cross-sectional studies, animal studies, commentary paper,
letters to the editor, and monographs were excluded from this metaanalysis.
Data extraction and quality assessment
The following data were extracted from each study: the last name
of first author, publication year, study location, study design, source
of population in cohort studies and the controls in case-control
studies, study sample size (number of cases and controls or cohort
size), exposures of interest, and target of HRT. The quality of eligible
studies was appraised by following the guidelines of the modified
Newcastle-Ottawa Quality Assessment Scale (NOS) documents.
Statistical analysis
Exposure to OC and exposure to HRT were analyzed as
dichotomous variables (ever used vs. never used). The data of exposed
cases, total exposed, unexposed cases, and total unexposed were
extracted and calculated using original data from the studies. These
data were then analyzed using the Mantel–Haenszel random-effects
method to calculate the RR with 95% CI. Statistical heterogeneity was
estimated by Cochran’s Q test and the I-squared test. The I- squared
test represents the percentage of variation that is attributable to
study heterogeneity, and it is categorized as low (0-40%), moderate
(40-60%), high (60-90%), and very high (>90%). Subgroup analysis
was carried out based on study location, study design, population
source, study sample size, exposures of interest, and target of HRT.
Heterogeneity between subgroups was evaluated by meta-regression.
Sensitivity analyses were performed by omitting one study at a time
to check if the inclusion criteria affected the final results. The Begg’s
test and Egger’s test were used to identify any potential publication
bias. All statistical analyses were performed using Review Manager
5.2 and STATA 12.0.
Table 1
Table 2
Table 3
Figure 2
Table 4
Results
Systematic review
We identified 3651 studies that fit our search strategy, but only
21 studies matched with selection criteria (Figure 1). After reviewing
full text, 2 studies were excluded because of duplicate report on the
same population [20,21], 3 studies were excluded because of ineligible
study subjects [22-24], and 4 studies were excluded because of the
lack of sufficient information to calculate effect estimates [7, 25-27].
Finally, 12 studies were selected for the analysis in this paper [8-19].
Detailed characteristics of these studies were summarized in
Table 1. The included studies were published between 1992 and 2013,
consisting of 6 case-control studies [8,9,11,14,16,17] and 6 cohort
studies [10,12,13,15,18,19]. These studies overall included 2805
women with pancreatic cancer and 759502 female controls [13-19].
Six studies were performed in North America [10-13,16,19], three
studies in Europe [8,17,18], two studies in China [9,15], and one as
a multicenter study in North America, Europe and Australia [14].
Eight comparison groups of these studies were population-based
[8,9,11-15,17], two studies were hospital-based [16,17], one was a
study for public school professionals [19], and one was for nurses
[10]. All selected studies addressed OC exposure [8-19], among which
10 studies had the data of HRT exposure [8,10-14,16-19]. However,
more than 20% of HRT exposure data were missing in the paper of
Skinner et al. [10], so it was excluded. For the HRT exposure, the
data of 4 studies were only on postmenopausal women [11-13,19],
and the other 5 studies were performed for both premenopausal and
postmenopausal women [8,14,16-18].
The NOS quality points of cohort studies are summarized in
Table 2, and the case-control studies were summarized in Table 3.
With NOS quality point >6 being defined as high quality and ≤6 as
low quality, we found that all of included studies were at high quality.
The effects of oral contraceptives on pancreatic cancer
risk
In the 12 included studies that examined the relationship between
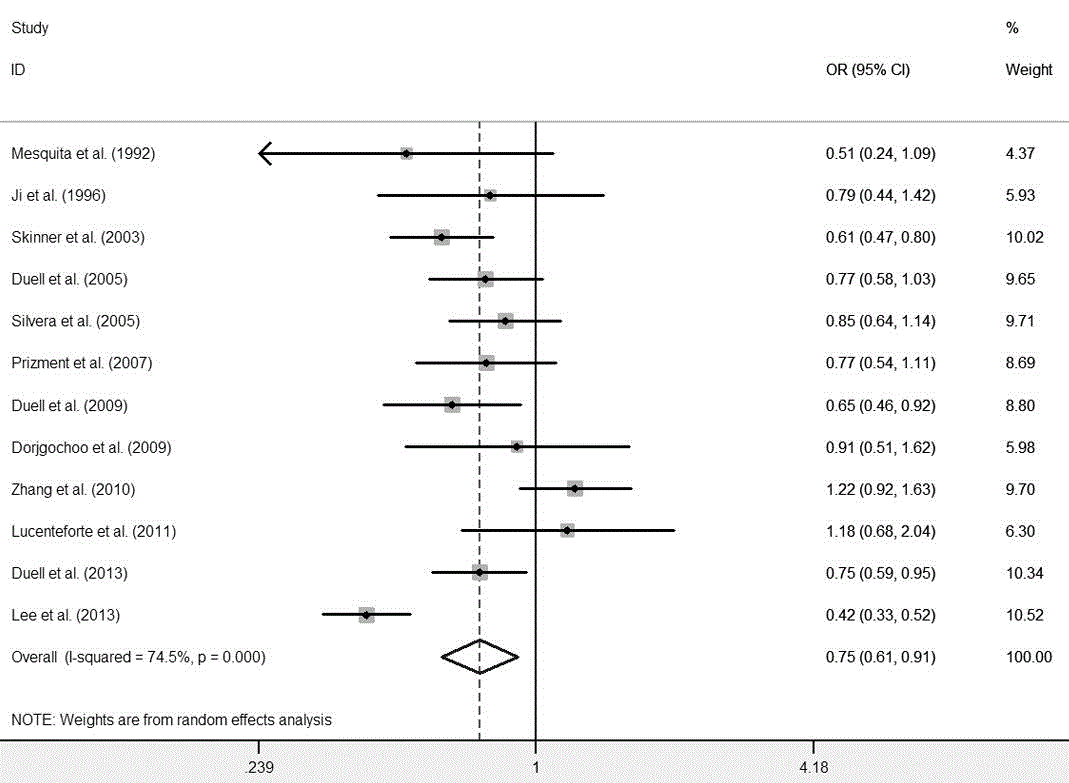
OC and pancreatic cancer risk [8-19], the study-specific ORs for OC
use ranged from 0.42 to 1.22. The pooled OR of pancreatic cancer
for OC ever used vs. never used was 0.75 (95% CI 0.61-0.91) with
significant heterogeneity (I2 = 74.5%, P=0.000) (Figure 2). There
was no indication of publication bias with Begg’s test (P = 1.000) or
Egger’s test (P = 0.723).
We further carried out a subgroup analysis. As shown in Table
4, when grouped by source of control subjects, OC use in population
based studies was associated with significant decrease in pancreatic
cancer risk (OR = 0.76, 95% CI 0.67-0.86) with less heterogeneity (I2
= 0.0%, p = 0.899). However, OC use in hospital based studies was
associated with insignificant increase in pancreatic cancer risk (OR
was 1.21, 95% CI 0.94-1.57) with less heterogeneity (I2 = 0.0%, p =
0.899). The association between OC use and pancreatic cancer risk
remained statistically significant in cohort studies and large cases
studies (n >200). The relationships between OC and pancreatic
cancer risk in case-control studies, small cases studies (n <200), Asia
patients studies, North America studies and Europe studies were all
not significant. There were some studies on the association between
the duration of OC use and pancreatic cancer risk. However, since
the length of duration is different, we cannot conduct a meta-analysis
on this subgroup.
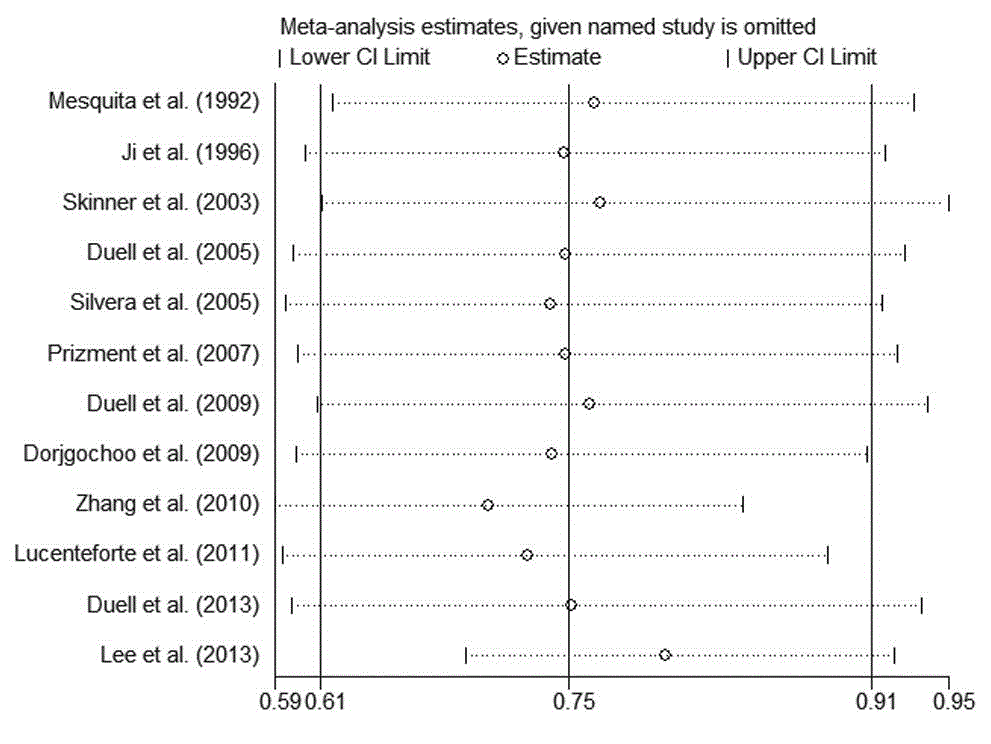
In sensitivity analyses, we sequentially removed one study at a time
and reanalyzed the data to examine the origin of heterogeneity. We
found that no single study altered the original results or heterogeneity
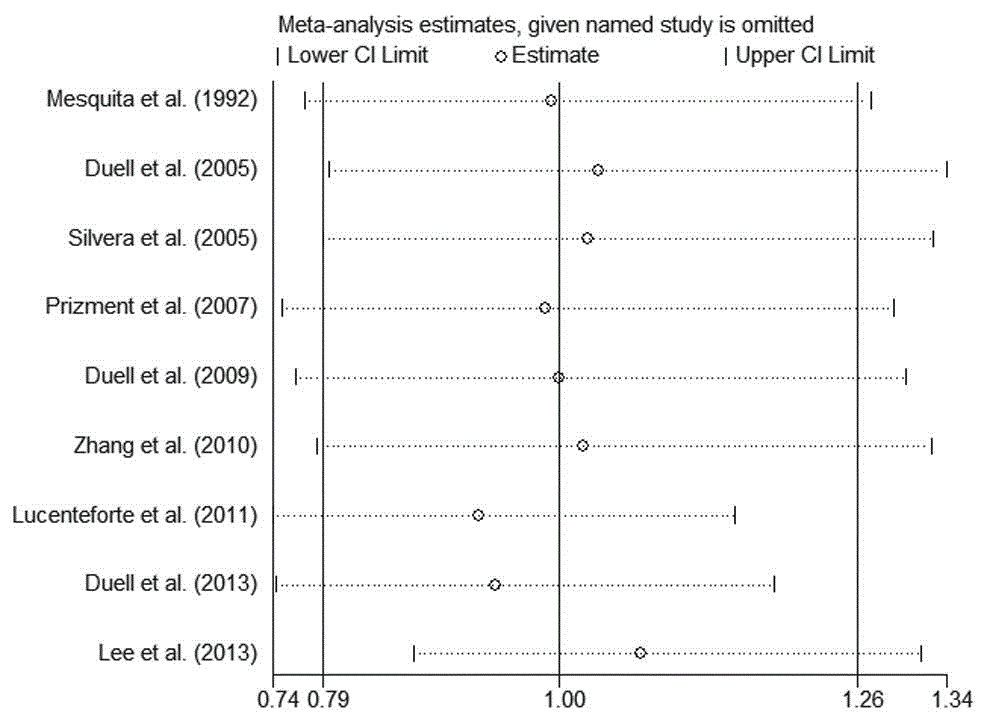
significantly (Figure 3).
The effects of hormone replacement therapy on pancreatic
cancer risk
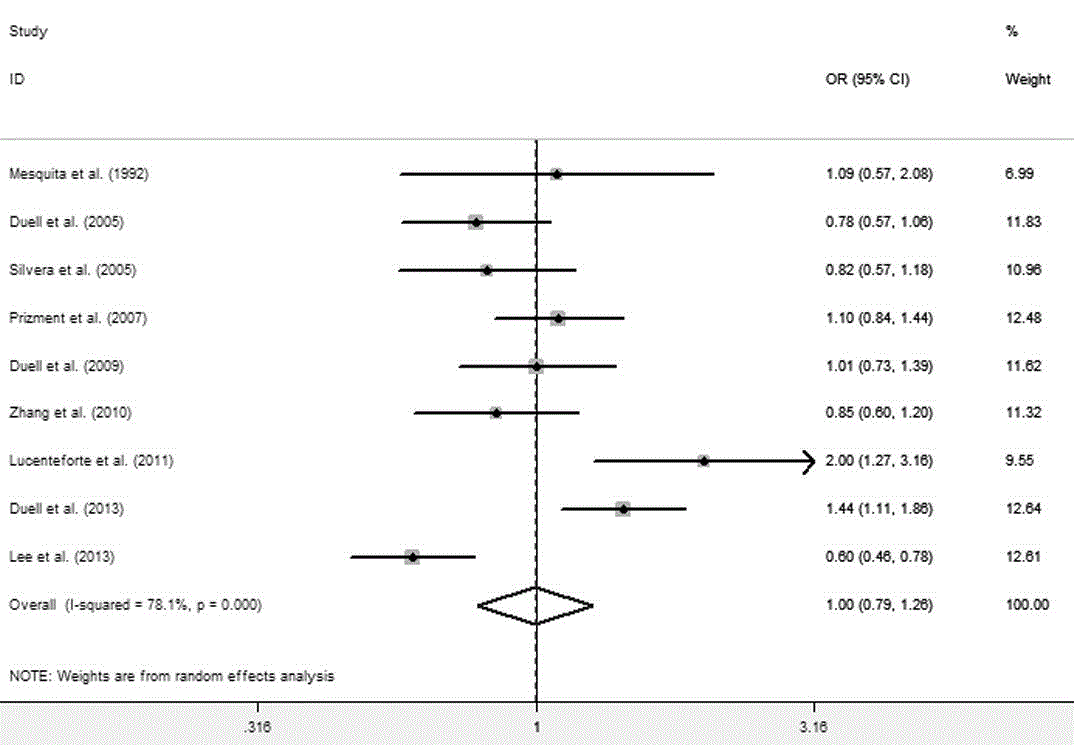
Nine studies were included in this study to examine the
association between HRT and pancreatic cancer risk [8,11-14,16-19].
The study-specific ORs for HRT use ranged from 0.6 to 2.00. The OR
of pancreatic cancer for HRT ever used vs. never used was 1.00 (95%
CI 0.79-1.26) with significant heterogeneity (I2 = 78.1% P=0.000)
(Figure 4). There was no indication of publication bias with Begg’s
test (P = 0.677) or Egger’s test (P = 0.951).
To reveal more detailed information, a subgroup analysis was
performed again with the results shown in Table 5. Studies based on
the European population showed that HRT significantly increased
pancreatic cancer risk (OR = 1.51, 95% CI 1.16-1.97) with less
heterogeneity (I2 = 22.7%, p = 0.274). However, studies based on the
North American population showed an opposite outcome (OR = 0.81,
95% CI 0.65-1.01) with larger heterogeneity (I2 =61.6%, p= 0.034).
In addition, HRT for postmenopausal women was associated with
decreased risk of pancreatic cancer (OR = 0.80, 95% CI 0.61-1.06).
However, HRT for both premenopausal and postmenopausal women
was associated with increased risk (OR =1.21, 95% CI 0.91-1.62). The
relationships between HRT and pancreatic cancer risk in case-control
studies, cohort studies, large cases studies, small cases studies were all
not significant. The durations of HRT were also different in different
studies, thus making it difficult to perform a meta-analysis on this
subgroup.
In sensitivity analyses, we found that no single study altered the
original results or heterogeneity significantly (Figure 5).
Discussion
To our knowledge, this is the first systematic review and metaanalysis
about the relationship between OC and HRT and pancreatic
cancer risk in women. In this meta-analysis, we strictly selected 12
studies, including 2805 pancreatic cancer patients and 759502 female
controls from Asia, Europe and North America. Overall, we found
that OC use was associated with significant decrease in pancreatic
cancer risk, but HRT showed no effect on pancreatic cancer risk or
generated controversial results.
Among many different etiology factors, the functions of steroid
hormones in pancreatic cancer development are not well studied.
However, the fact of hormonal imbalance in pancreatic cancer
incidence with a male to female ratio ranging from 1.25-1.75:1
[28] suggests the importance of hormonal regulation in pancreatic
cancer development. Indeed, ERα and ERβ have been identified to
be differentially expressed in pancreatic cancer with ERβ playing
even more important function [29]. Estrogen has been known to
play a wide range of functions from development, regulation of
reproductive system [30-33] to cancer development [34], especially
in breast and ovarian cancers [35]. Thus, it is significant to conduct
this study to help clarify the relationship between the OC use and
HRT and pancreatic cancer development. Since both OC and
HRT are composed of various synthetic estrogens and progestins
at different doses that are more likely to perturb the endogenous
hormonal balance, the potential to cause or prevent pancreatic cancer
development is then very high. While it is a difficult topic at basic
research level, epidemiologic study could shed some light first on
their relationship with pancreatic cancer.
During the meta-analysis of the relationship between OC use and
pancreatic cancer risk, since there was no significant publication bias,
but showing high heterogeneity, we further carried out a subgroup
analysis to identify factors leading to the heterogeneity. Limited by
the numbers of studies, insufficient data, different adjusting factors, it
was difficult to do subgroup analyses on duration, dose-response and
potential confounding adjustment. Furthermore, our study identified
that cohort studies showed a lower risk of pancreatic cancer by OC use
than case-control studies. While OC use was found to be associated
with significant decrease in pancreatic cancer risk in population
based studies, hospital based studies showed an opposite results.
Besides, these two groups of studies showed homogeneity (I2 = 0.0%),
thus providing a strong evidence that OC use was associated with the
decreased risk of pancreatic cancer in general population. The reason
why OC can decrease pancreatic cancer risk is partially derived from
the fact that OC can change the endogenous estrogen signaling,
therefore leading to the promotion or inhibition of cancer progression.
ER signaling has been known to either promote or inhibit cancer cell
proliferation at lower dose or at higher dose, respectively [29]. Thus,
the actual levels of estrogen in women who take OC are dramatically
diverse that lead to a wide range of outcomes. In addition, estrogen
signaling is also related with adipose tissue metabolism and obesity
that is one of main risk factors for pancreatic cancer [3]. The reason
for the increased pancreatic cancer risk in hospitalized population is
unknown. However, the compromised immune system could be one
of reasons.
Our meta-analysis revealed that HRT was generally no effect
on pancreatic cancer risk. The selected studies had no significant
publication bias, but showing high heterogeneity. Again, a subgroup
analysis was performed, which showed that cohort studies had a lower
risk than case-control studies; population based studies had a lower
risk than hospital based studies. However, both differences were
not significant. Studies showed that HRT in premenopausal women
increased pancreatic cancer risk; whereas HRT in postmenopausal
women decreased pancreatic cancer risk. This may be due to the
estrogen level difference between premenopausal and postmenopausal
women, thus HRT causes the estrogen level in premenopausal
women higher than physiological range, leading to the increased risk
of pancreatic cancer development. Our meta-analysis also identified
that HRT was associated with decreased pancreatic cancer risk in
North American population but with increased risk in European
population. It is not clear what are the exact reasons leading to these
differences, but many factors, such as life style, higher diversity of
North American population, and different brands of HRT, may be
attributed to the different outcomes.
Figure 3
Figure 3
Influence of individual studies on the pooled OR in meta-analysis of OC and pancreatic cancer risk.
Figure 4
Figure 5
Figure 5
Influence of individual studies on the pooled OR in meta-analysis of HRT and pancreatic cancer risk.
Table 5
Conclusion
This systematic review and meta-analysis provides further evidence that OC use is associated with decreased risk of pancreatic cancer in general women population; in contrast HRT shows no effect in overall studies. Because of the fast progression and poor prognosis features of pancreatic cancer and the lack of early diagnosis methods, it is extremely difficult to collect accurate information from participants in epidemiological or clinical studies of pancreatic cancer. With additional complexity from a wide range of types and formulations of OC and HRT used all over the world, the analysis of the relationship between OC or HRT and pancreatic cancer risk becomes an even more difficult task. Thus, further large scale and well controlled studies are needed to finally elucidate specific relationship between OC or HRT and the risk of pancreatic cancer in different populations or associated with other pancreatic cancer risk factors.
Acknowledgement
This study was supported by National Natural Science Foundation of China (No. 81001113). We thank Ms. Kriszta Farkas, MPH (Epidemiologist at University of California, Berkeley) for her critical comments.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63: 11-30.
- La Vecchia C, Bosetti C, Lucchini F, Bertuccio P, Negri E, Boyle P, et al. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann Oncol. 2010; 21: 1323-1360.
- Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir. 2004; 59: 99-111.
- Nacusi LP, Debes JD. Primers on molecular pathways: nuclear receptors in pancreatic cancer. The ligand-independent way. Pancreatology. 2008; 8: 422-424.
- Polvani S, Tarocchi M, Tempesti S, Galli A. Nuclear receptors and pathogenesis of pancreatic cancer. World J Gastroenterol. 2014; 20: 12062-12081.
- Cogliano V, Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, et al. Carcinogenicity of combined oestrogen-progestagen contraceptives and menopausal treatment. Lancet Oncol. 2005; 6: 552-553.
- Adami HO, Persson , Hoover R, Schairer C, Bergkvist L. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer. 1989; 44: 833-839.
- Bueno De Mesquita HB, Maisonneuve P, Moerman CJ, Walker AM. Anthropometric and reproductive variables and exocrine carcinoma of the pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1992; 52: 24-29.
- Ji BT, Hatch MC, Chow WH, McLaughlin JK, Dai Q, Howe GR, et al. Anthropometric and reproductive factors and the risk of pancreatic cancer: a case-control study in Shanghai, China. Int J Cancer. 1996; 66: 432-437.
- Skinner HG, Michaud DS, Colditz GA, Giovannucci EL, Stampfer MJ, Willett WC, et al. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003; 12: 433-438.
- Duell EJ, Holly E. A. Reproductive and menstrual risk factors for pancreatic cancer: A population-based study of San Francisco Bay Area women. Am J Epidemiol. 2005; 161: 741-747.
- Navarro Silvera SA, Miller AB, Rohan TE. Hormonal and reproductive factors and pancreatic cancer risk: a prospective cohort study. Pancreas. 2005; 30: 369-374.
- Prizment AE, Anderson KE, Hong CP, Folsom AR. Pancreatic cancer incidence in relation to female reproductive factors: Iowa Women's Health Study. J Pancreatology. 2007; 8: 16-27.
- Duell EJ, Maisonneuve P, Baghurst PA, Bueno-de-Mesquita HB, Ghadirian P, Miller AB, et al. Menstrual and reproductive factors and pancreatic cancer in the SEARCH program of the IARC. Cancer Causes Control. 2009; 20: 1757-1762.
- Dorjgochoo T, Shu XO, Li HL, Qian HZ, Yang G, Cai H, et al. Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer. 2009; 124: 2442-2449.
- Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. A case-control study of reproductive factors, female hormone use, and risk of pancreatic cancer. Cancer Causes and Control. 2010; 21: 473-478.
- Lucenteforte E, Zucchetto A, Bosetti C, Talamini R, Negri E, Serraino D, et al. Reproductive and hormonal factors and pancreatic cancer risk in women. Pancreas. 2011; 40: 460-463.
- Duell EJ et al. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer. 2013; 132: 2164-2175.
- Lee E, Horn-Ross PL, Rull RP, Neuhausen SL, Anton-Culver H, Ursin G, et al. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol. 2013; 178: 1403-1413.
- Fernandez E, La Vecchia C, D'Avanzo B, Negri E. Menstrual and reproductive factors and pancreatic cancer risk in women. Int J Cancer. 1995; 62: 11-14.
- Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int J Cancer. 2003; 105: 408-412.
- Teras LR, Patel AV, Rodriguez C, Thun MJ, Calle EE. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. Women (United States). Cancer Causes and Control. 2005; 16: 1035-1040.
- Lin Y, Kikuchi S, Tamakoshi A, Kawamura T, Inaba Y, Kurosawa M, et al. Association of menstrual and reproductive factors with pancreatic cancer risk in women: Findings of the Japan collaborative cohort study for evluation of cancer risk. J Gastroenterol. 2006; 41: 878-883.
- Lo AC, Soliman AS, El-Ghawalby N, Abdel-Wahab M, Fathy O, Khaled HM, et al. Lifestyle, occupational, and reproductive factors in relation to pancreatic cancer risk. Pancreas. 2007; 35: 120-129.
- Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy--long-term follow-up of a Swedish cohort. Int J Cancer. 1996; 67: 327-332.
- Kreiger N, Lacroix J, Sloan M. Hormonal factors and pancreatic cancer in women. Ann Epidemiol. 2001; 11: 563-567.
- Rosenblatt KA, Dao L. Gao, Roberta M. Ray, Zakia C. Nelson, Karen J. Wernli, Wenjin Li, et al. Oral contraceptives and the risk of all cancers combined and site-specific cancers in Shanghai. Cancer Causes and Control. 2009; 20: 27-34.
- Imamura Y, Mizuno S. Comparison of pancreatic cancer mortality in five countries: France, Italy, Japan, UK and USA from who mortality database (1960-2000). Jpn J Clin Oncol. 2005; 35: 283-286.
- Iwao K, Miyoshi Y, Ooka M, Ishikawa O, Ohigashi H, Kasugai T, et al. Quantitative analysis of estrogen receptor-alpha and -beta messenger RNA expression in human pancreatic cancers by real-time polymerase chain reaction. Cancer Lett. 2001; 170: 91-97.
- Moss RL, Gu Q, Wong M. Estrogen: nontranscriptional signaling pathway. Recent Prog Horm Res. 1997; 52: 33-68.
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007; 47: 657-680.
- Fucic A, Miskov S, Zeljezić D, Bogdanovic N, Katić J, Gjergja R, et al. Is the role of estrogens and estrogen receptors in epilepsy still underestimated? Med Hypotheses. 2009; 73: 703-705.
- Fucic A, Gamulin M, Ferencic Z, Katic J, Martin Krayer von Krauss, Bartonova A, Domenico F Merlo. Environmental exposure to xenoestrogens and oestrogen related cancers: reproductive system, breast, lung, kidney, pancreas, and brain. Environ Health. 2012; 11: S8.
- Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Med Res Rev. 2008; 28: 954-974.
- Brown SB, Hankinson SE2. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2015; 99(Pt A): 8-10.