Research Article
A Systematic Review of Concordance between Indocyanine Green and 99m Technetium Sentinel Lymph Node Identification in Melanoma
Stephanie L. Koonce*, Martin I. Newman
University of Minnesota Medical School, USA
*Corresponding author: Stephanie L. Koonce, University of Minnesota Medical School, Cleveland Clinic Florida, 2950 Cleveland Clinic Blvd., Weston, USA
Published: 23 Jun, 2017
Cite this article as: Koonce SL, Newman MI. A Systematic
Review of Concordance between
Indocyanine Green and 99m
Technetium Sentinel Lymph Node
Identification in Melanoma. Clin Oncol.
2017; 2: 1308.
Abstract
Introduction: Radiocolloid 99mTechnetium with or without blue dyes is the most commonly
employed method of identifying Sentinel Lymph Nodes (SLN) in melanoma staging. Indocyanine
green (ICG) identification of SLN has been reported with equal or superior results and could avoid
the use of a radioactive tracer. A systematic review and meta-analysis of the literature was performed.
Methods: A systematic review of the literature was performed identifying peer-reviewed articles
examining the concordance between 99mTc and ICG in the identification of SLN in individuals
undergoing SLN biopsy for melanoma. Only original study groups were included. The SLN false
negative rate and identification rate were pooled according to radiocolloid, blue dye, ICG, or a
combination.
Results: Between 1990 and 2016 a total of 14 studies were reported which met inclusion criteria.
These studies included a total of 456 patients. The pooled SLN identification rate in patients
using radiocolloid with or without blue dye was 1.94 (range 1.14-2.7). Using ICG the pooled SLN
identification rate was 2.11 (range 1.1-2.67).
Conclusions: Systematic review of the literature demonstrates no significant difference in SLN
identification rate between ICG and 99mTc in melanoma patients. A prospective, randomized
controlled trial comparing ICG to 99mTc to more definitively establish ICG as an alternative
modality for SLN identification is recommended.
Introduction
Melanoma is the leading cause of skin cancer mortality and is diagnosed annually in 114,000
patients [1]. Melanoma metastasizes primarily via the lymphatic route with the first lymph nodes
metastatic cells encounter being designated the Sentinel Lymph Nodes (SLN). Sentinel Lymph Node
Biopsy (SLNB) is recommended by the National Comprehensive Cancer Network for staging of
all melanomas with a breslow depth greater than 1 mm and may be considered in patients with
melanoma depths less than 1 mm with high risk features [2].
The identification of SLN in melanoma patients is most commonly performed with a
combination of blue dye and radiocolloid tracer. An injection of technetium 99m (99mTc) is
instilled intradermally around the primary tumor site. Imaging follows, and intra-operative use
of a handheld gamma probe allows localization, identification, and confirmation upon extirpation
of the node. The blue dye is injected intradermally around the primary tumor site immediately
prior to surgery. The blue dye and 99mTc act complementarily in identification of the SLN. SLN
identification using these two methods has a reported success rate of 96-99% [3].
Indocyanine green (ICG) is a cyanine dye first tested in 1957 at the Mayo Clinic for used in human
medicine [4]. The Federal Drug Administration has approved it for use in medical diagnostics. ICG
binds tightly to plasma proteins, is hepatically microsomally metabolized, and has a half-life of 150-
180 seconds [5]. Side effects are rare and mostly minor (including flushing and sore throat).
A systematic review was performed on peer-reviewed articles to evaluate the rates of SLN
identification with ICG versus radiocolloid, blue dye, or a combination of radiocolloid and blue dye.
In addition, we report our technique for ICG identification of SLN in melanoma patients with SLN
in known lymph node basins.
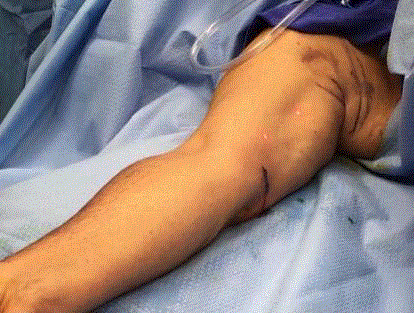
Figure 1
Figure 1
ICG is injected intradermally in four equal aliquots at the primary
tumor site prior to incision.
Figure 2
Figure 2
Near infra-red (NIR) imaging is performed to obtain fluorescence
imaging of the subcutaneous lymphatics.
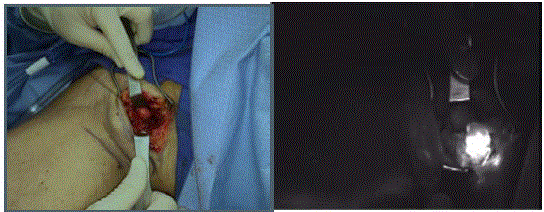
Figure 3
Figure 3
Once the lymph node basin is identified, an access incision is
performed The lymph node basin is then gently and bluntly probed while
visualizing the soft tissue under NIR guidance.
Methods
A comprehensive systematic review of published literature
over the years 1990-2016 was conducted using the search terms:
“melanoma”, “sentinel lymph nodes”, “lymphatic mapping”,
“lymph node mapping”, “radiocolloid”, “technetium”, “ICG”, and
“indocyanine” in MEDLINE and EMBASE. The search was then
supplemented with the references of the selected articles. Inclusion
criteria included original study population and intraoperative
identification of sentinel lymph node using ICG, radiocolloid with
or without blue dye, or a combination of techniques. Studies were
required to report the number of patients in whom SLNB were
attempted and the number who were successfully mapped with each
technique. Studies that did not report SLN identification rate were
excluded. Data from all included studies was analyzed with Review
Manager 5.3 (Cochrane Review 2014). Data was verified between two
reviewers and discrepancies were settled by consensus discussion.
Quality of the studies was evaluated using the Quality Assessment
of Diagnostic Accuracy Studies (QUADAS) tool. Each item on the
QUADAS tool was recorded as “yes”, “no”, or “unclear”.
Outcomes were noted as measures of test performance: the
proportion of lymph nodes successfully mapped with ICG and
the proportion of lymph nodes successfully mapped with 99mTc.
Distributions of covariates were evaluated and summary measures of
central tendency and variability were estimated. Summary measures
of all outcome measures across studies were estimated by the Mantel-
Haenszel method. As significant heterogeneity was observed for
most outcomes, a random effects model was utilized with each study
weighted by the inverse of its variance. Summary effect estimates
hypothesis testing was based on the z-statistic with 95% CIs provided
for individual studies, as well as the summary overall effect estimate.
Method of ICG SLN identification
Although methods vary among surgeons, our methods are
outlined here for reference. This method is most easily employed
when the sentinel lymph node is expected to reside in a specific lymph
node basin (e.g.: axilla or groin) though pre-operative knowledge
of the nodal basin is not required. After intubation, the patient is
prepped and draped in the normal sterile fashion. Using a 30 gauge
needle and a 1 cc syringe, a total of 0.10 ml of 500 uM ICG is injected
intradermally in four equal aliquots at the primary tumor site prior to
incision (Figure 1) Larger lesions may require the injection of a second
aliquot. It should be noted that injecting intradermal quantities of
ICG in excess of this amount might overwhelm the lymphatic basin
resulting in a complete “white out” when visualized under near infrared
(NIR) imaging. After ICG injection, the surgeon should wait
at least ten minutes before excising the tumor or manipulating the
lymphatic channels to allow the ICG to travel to the SLN. Near infrared
(NIR) imaging is performed to obtain fluorescence imaging of
the subcutaneous lymphatics (Figure 2). Once the lymph node basin
is identified, an access incision is performed (Figure 3). The lymph
node basin is then gently and bluntly probed while visualizing the
soft tissue under NIR guidance. In our experience, the SLN is easily
visualized using this method. Once identified in situ, it is excised.
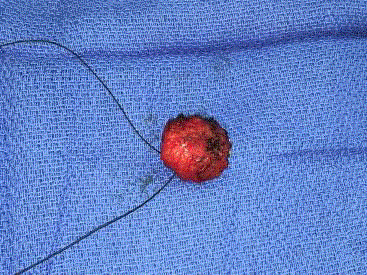
Confirmation of the node fluorescence is made with NIR after the
excision (Figure 4 and 5).
Figure 4
Figure 5
Figure 6
Figure 7
Results
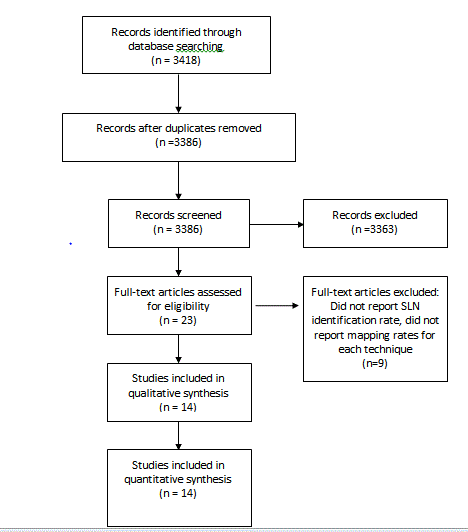
The original literature search returned 3418 references. After
excluding abstracts that did not meet inclusion criteria or were unable
to be translated into English, 23 abstracts were extracted for full text
review (Figure 6). Of these, 14 met criteria for full analysis (Table 1).
Years of publication of analyzed studies ranged from 2009 to
2016. After aggregation of all data, 456 patients underwent SLNB
for melanoma with indocyanine green. In addition to the ICG,
449/456 patients also had technetium radioactive tracer used as a
control for SLN identification; 263/456 also had blue dye used as
a second control for SLN identification. In studies that reported
gender, 45.4% (139/306) were female. Follow-up duration averaged
5.6 months (range 3.2-9 months). The primary tumor was localized
on the extremities, trunk, or head/neck in 50.6%, 35.9%, and 13.5%
respectively. Mean tumour depth was 2.22 mm (range 0.9-3.73 mm).
A blue dye was also used for SLN mapping in 263 of the 456 patients.
Blue dyes utilized included methylene blue, nonvital blue dye, and
isosulfan blue. The mean number of SLN removed identified by ICG
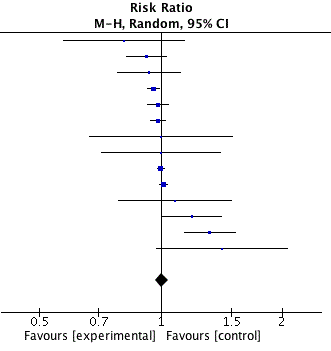
was 2.11 and 1.94 for 99mTc with or without blue dye. No significant
difference was found between identification rates of SLN with ICG
versus 99mTc with or without blue dye (p <0.00001) (Figure 7).
Pooled false negative rate for ICG was 2.8% and 5.3% for 99mTc.
Discussion
Sentinel lymph node biopsy is an established diagnostic
procedure in melanoma staging to detect subclinical lymph node
metastases. A survival benefit has been demonstrated in patients who
underwent lymphadenectomy for intermediate thickness melanoma
with positive SLN compared to patients who were observed with
lymph node dissection once nodal disease was evident [6]. SLNB
is an important staging tool, but has not been shown to improve
disease specific survival among all patients. However, among patients
with melanoma thickness of 1.2 to 3.5 mm, SLNB is associated with
improvement in distant metastasis free survival compared to patients
with similar thickness melanoma who are initially observed and
subsequently develop nodal metastases [2]. Over the past 18 years,
identification of SLN has improved to an overall rate greater than
95% [3]. Reported false negative rate is approximately 5-10% [6,9-
22]. False negatives may occur for myriad reasons including nonlocalization
of the SLN. ICG was found to have a lower false negative
rate than 99mTc in this review.
Blue dyes have been associated with allergic reactions including
urticaria, hives, skin rash, and severe anaphylactic shock [21]. The
rate of anaphylaxis was reported as 0.5% to isosulfan blue in a series
of 2392 SLN with an overall allergic reaction incidence of 1.6% [23].
Blue dye injection can also cause permanent skin tattooing and stains
the surrounding tissue obscuring the surgical field. It is currently
contraindicated in pregnancy. 99mTc requires an additional clinic
or hospital visit, exposure to radiation, a frequently painful injection,
and not insignificant cost. Radiotracer mapping is limited in most
developing countries, and production constraints with possible
shortage of 99mTc are predicted [22].
Indocyanine green is a fluorescent dye visible with near infrared
light that represents an alternative to 99mTc and blue dyes. The side
effect profile is minimal with one series reporting 0.15% mild reactions
and 0.05% severe reactions [4,5,24]. The small molecular size of ICG
compared to the radiocolloids allows for uptake in lymphatics that
may be partially blocked from tumor, previous surgery, or trauma.
This could explain why ICG may decrease the false negative SLN rate
by detecting additional SLN. Fujisawa and Jain noted a higher average
number of SLN removed with ICG compared to 99mTc, but other
studies noted no significant difference [7-10].
Optimal dosage of ICG for SLN identification has been
investigated using dose-escalating protocols. A comparison of 600,
800, 1000, and 1200 uM of ICG: HSA (albumin bound ICG) for SLN
identification found 600uM to be the optimal dose [11]. A lower dose
study comparing 100, 250, and 500uM ICG:HSA determined 500uM
provided the best SLN identification [12]. This suggests that dosage
should be in the 500 uM-600 uM range.
The surgeon is able to follow lymphatic drainage in real time at
time of ICG injection. The fluorescent nodes themselves, however,
are only visible after the skin incision is made, thus the nodal basin
should be known prior to injection particularly if the patient has
a high BMI, the suspected nodal basin is deep, or there is variable
lymphatic drainage [13,14].
Systematic review of the literature demonstrates SLN
identification rate is similar between ICG and 99mTc in melanoma
patients. Given the predicted shortage and high cost of 99mTc, ICG
appears to be a viable option for SLN identification in expected
lymph node basins at facilities with limited or no access to radiotracer
lymphoscintigraphy. The review suggests that a hybrid approach
using both ICG and 99mTc might be the best of both worlds by
producing the lowest false negative rate and allowing pre-operative
identification of the nodal basin of interest. A hybrid tracer of ICG
and 99mTc is being studied which would allow basin identification
pre-operatively but would not obviate the issues with 99mTc [15,16].
ICG is well tolerated with a reasonable side effect profile. Further
study with a prospective, randomized controlled trial comparing ICG
to 99mTc is recommended to more definitively establish ICG as an
alternative modality for SLNB in select cases.
Table 1
References
- World Cancer Research Fund International.
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Melanoma Version 3; 2016.
- Niebling MG, Pleijhuis RG, Bastiaannet E, Brouwers AH, van Dam GM, Hoekstra HJ. A systematic review and meta-analyses of sentinel lymph node identification in breast cancer and melanoma, a plea for tracer mapping. Eur J Surg Oncol. 2016; 42: 466-473.
- Fox JJ, Brooker L, Heselstine D. A tricarbocyanine dye for continuous recording of dilution curves in the whole blood independent of variations in blood oxygen saturation. Proc Staff Meeting Mayo Clinic. 1957; 32: 478-484.
- Ketterer SG, Wiengand BD: Hepatic clearance of indocyanine green. Clin Res. 1959; 7: 289-292.
- Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006; 355: 1307-1317.
- Korn JM, Tellez-Diaz A, Bartz-Kurycki M, Gastman B. Indocyanine green SPY elite-assisted sentinel lymph node biopsy in cutaneous melanoma. Plast Reconstr Surg. 2014; 133: 914-922.
- Jain V, Phillips BT, Conkling N, Pameijer C. Sentinel lymph node detection using laser-assisted indocyanine green dye lymphangiography in patients with melanoma. Int J Surg Oncol. 2013; 2013: 904214.
- Stoffels I, von der Stück H, Boy C, Pöppel T, Körber N, Weindorf M, et al. Indocyanine green fluorescence-guided sentinel lymph node biopsy in dermato-oncology. J Dtsch Dermatol Ges. 2012; 10: 51-57.
- Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. A custom-made, low-cost intraoperative fluorescence navigation system with indocyanine green for sentinel lymph node biopsy in skin cancer. Dermatology. 2011; 222: 261-268.
- van der Vorst JR, Schaafsma BE, Verbeek FP, Swijnenburg RJ, Hutteman M, Liefers GJ, et al. Dose optimization for near-infrared fluorescence sentinel lymph node mapping in patients with melanoma. Br J Dermatol. 2013; 168: 93-98.
- Gilmore DM, Khullar OV, Gioux S, Stockdale A, Frangioni JV, Colson YL, et al. Effective low-dose escalation of indocyanine green for near-infrared fluorescent sentinel lymph node mapping in melanoma. Ann Surg Oncol. 2013; 20: 2357-2363.
- Cloyd JM, Wapnir IL, Read BM, Swetter S, Greco RS. Indocyanine green and fluorescence lymphangiography for sentinel lymph node identification in cutaneous melanoma. J Surg Oncol. 2014; 110: 888-892.
- Namikawa K, Tsutsumida A, Tanaka R, Kato J, Yamazaki N. Limitation of indocyanine green fluorescence in identifying sentinel lymph node prior to skin incision in cutaneous melanoma. Int J Clin Oncol. 2014; 19: 198-203.
- Frontado LM, Brouwer OR, van den Berg NS, Mathéron HM, Vidal-Sicart S, van Leeuwen FW, et al. Added value of the hybrid tracer indocyanine green-99mTc-nanocolloid for sentinel node biopsy in a series of patients with different lymphatic drainage patterns. Rev Esp Med Nucl Imagen Mol. 2013; 32: 227-233.
- Brouwer OR, Buckle T, Vermeeren L, Klop WM, Balm AJ, van der Poel HG, et al. Comparing the hybrid fluorescent-radioactive tracer indocyanine green-99mTc-nanocolloidwith 99mTc-nanocolloid for sentinel node identification: a validation study using lymphoscintigraphy and SPECT/CT. J Nucl Med. 2012; 53: 1034-1040.
- Rubinstein TJ, Perry JD, Korn JM, Costin BR, Gastman BR, Singh AD. Indocyanine green-guided sentinel lymph node biopsy for periocular tumors. Ophthal Plast Reconstr Surg. 2014; 30: 301-304.
- Stoffels I, Dissemond J, Pöppel T, Schadendorf D, Klode J. Intraoperative Fluorescence Imaging for Sentinel Lymph Node Detection: Prospective Clinical Trial to Compare the Usefulness of Indocyanine Green vs Technetium Tc 99m for Identification of Sentinel Lymph Nodes. JAMA Surg. 2015; 150: 617-623.
- Fujiwara M, Mizukami T, Suzuki A, Fukamizu H. Sentinel lymph node detection in skin cancer with patietns using real-time fluorescence navigation with indocyanine green: a preliminary experience. J Plast Reconstr Aesthet Surg. 2009; 62: 373-378.
- Polom K, Murawa D, Rho YS, Spychala A, Murawa P. Skin melanoma sentinel lymph node biopsy using real-time fluorescence navigation with indocyanine green and indocyanine green with human serum albumin. Br J Dermatol. 2012; 166: 682-683.
- Dewachter P, Mouton-Faivre C, Tréchot P, Lleu JC, Mertes PM. Severe anaphylactic shock with methylene blue instillation. Anesth Analg. 2005; 101: 149-150.
- Nuclear Energy Agency Organisation for Economic Co-operation and Development. The supply of medical radioisotopes: an economic study of the molybdenum-99 supply chain. 2010.
- Montgomery LL, Thorne AC, Van Zee KJ, Fey J, Heerdt AS, Gemignani M, et al. Isosulfan blue dye reactions during sentinel lymph node mapping for breast cancer. Anesth Analg. 2002; 95: 385-388.
- Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994; 101: 529-533.
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6: e1000097.