Mini Review
Neurolymphomatosis: Treatment Response and Progression Monitored by 18F-FDG PET/CT
Pierre Abouhamad1, Roger Denays2*, Vincent Baudrez3, Jasmine Nguyen4, Thierry Dereme1 and Jean-Philippe Hermanne4
1Department of Nuclear Medicine, Rega Institute for Medical Research, Belgium
2Department of Neurology, Rega Institute for Medical Research, Belgium
3Department of Radiology, Rega Institute for Medical Research, Belgium
4Department of Hemato-Oncology, Rega Institute for Medical Research, Belgium
*Corresponding author: Roger Denays, Department of Neurology, Rega Institute for Medical Research, Belgium
Published: 14 Jun, 2017
Cite this article as: Abouhamad P, Denays R, Baudrez
V, Nguyen J, Dereme T, Hermanne
J-P. Neurolymphomatosis: Treatment
Response and Progression Monitored
by 18F-FDG PET/CT. Clin Oncol. 2017;
2: 1305.
Abstract
Neurolymphomatosis (NL)) is the term for infiltration of the peripheral nervous system by
neurotropic neoplastic cells in the setting of a known or unknown haematological malignancy.
Optimal treatment management of this rare clinical entity remains ill defined. We report the case
of a 65-year-old woman with plexiform NL of the right sciatic nerve. Initial complete response to
systemic chemotherapy, and later, relapse and progression, were accurately assessed by sequential
18-FDG-PET/CT studies, performed after a few cycles of the initial chemotherapy, and following
completion of both initial treatment and salvage therapy.
Keywords: Neurolymphomatosis; PET/CT; Response assessment
Introduction
A plexiform Neurolymphomatosis (NL) of the right sciatic nerve was diagnosed in a 65-yearold woman with progressive sensori-motor impairment of the right lower leg, diagnostic workup including MRI, 18-FDG-PET/CT and open biopsy of a FDG-avid inguinal adenopathy. MRI results have been previously reported elsewhere [1]. Baseline 18-FDG-PET/CT showed intense heterogenous tracer uptake along the right sciatic nerve, as well as in numerous nodal and extra nodal sites (Figure 1A and C). Histological analysis revealed an aggressive B-cell non-Hodgkin lymphoma, unclassifiable, intermediate between diffuse large B-cell lymphoma and Burkitt's lymphoma. Immuno-histochemical analysis showed that tumor cells were positive for CD20, CD79a, BCL2, BCL6, and negative for CD10, CD5, CD23, D1 cyclin, TDT, CD34. No translocation of cMYC gene was detected on fluorescent in situ hybridization analysis. The patient was treated by eight cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). Interim 18-FDG-PET/CT, performed after three R-CHOP cycles, detected no residual FDG-avid tumour (Figure 1D), contrasting with only partial neurological and MRI improvement. One month after the last chemotherapy cycle, the patient complained of reduced strength in the left hand and painless numbness from the fourth and fifth fingers to the ulnar styloid process, suggesting additional left ulnar nerve involvement. Left ulnar neuropathy was confirmed by electro diagnostic studies. Zoography of the left upper limb revealed a diffuse ulnar nerve thickening, consistent with NL 18-FDG-PET/CT showed discontinuous hyper metabolic uptake in the left ulnar nerve as well as tumoral relapse in the right sciatic nerve and in a multitude of nodal and extra nodal sites (Figures 2A and B). Zoographically-guided biopsy of a hyper metabolic lesion in the right mammary gland confirmed unclassifiable aggressive B-cell non-Hodgkin lymphoma. Laboratory tests showed mild red cell count decrease (3, 4 106/mm3) and increased serum LDH (277 U/l, N: 135-250)). Cerebrospinal fluid examination revealed high protein content (109 mg/dl, N: 15-45), low glucose level (28mg/ dl, N: 40-80) and a mild pleiocytosis (24 mm3, N: 0-10) that consisted mostly of lymphoma cells. Three cycles of R-DHAOx (rituximab, dexamethasone, high-dose cytarabine, oxaliplatin) and intrathecal injections of cytarabine, every 3 weeks, were administered. Unfortunately, response to salvage chemotherapy, as assessed by 18-FDG-PET/CT at the end of therapy, was only partial (SUV reduction ranging from 40% to 50%, depending on the involved site) and the patient was placed under supportive care. She died 15 months after her initial diagnosis of NL. NL is the term for infiltration of the peripheral nervous system by neurotropic neoplastic cells in the setting of a known or unknown haematological malignancy [2]. Early diagnosis of this rare clinical entity is now greatly facilitated by the use of contemporary imaging techniques, such as MRI and 18-FDG-PET/CT) [1-13]. To date, optimal therapeutic management of NL remains however ill defined [2-4] and the median survival (2 to 21 months from diagnosis as a function of the underlying haematological malignancy) [2,4] is still far shorter than in lymphomas without NL [14,15]. The majority of patients with NL now undergoes systemic chemotherapy alone or combined with intrathecal chemotherapy or radiotherapy, mainly limited-field radiotherapy to ensure complete tumour eradication or relieving unremitting neuropathic pain attributed to a particular nerve, plexus or nerve root lesion [2-4]. Assessing therapy response is particularly challenging in NL, as, even in absence of residual malignancy, neurological recovery and resolution of electrophysiological and MRI anomalies are often incomplete, probably reflecting persistent changes in neural and per neural structures [2]. Furthermore, in a patient who develops new neurological symptoms during or after therapy, NL relapse or progression has to be differentiated from more common non-tumour disorders associated with leukaemia or lymphoma such as nerve damage from reactivation of latent herpes zoster virus, lymphoma-associated vasculitis, chemotherapy-induced polyneuropathy, radiation plexopathy, or Guillain-Barre syndrome [2-4,9]. As suggested by the present case and by some previous reports, PET/CT is potentially more accurate than conventional imaging to monitor therapy response and NL progression, due to (1) complete normalization of FDG uptake in previously FDG-avid neural structures when therapy is effective [2,5,6,10-12]. (2) A superior sensitivity and specificity of PET/CT for the detection of NL [2,5-12], including asymptomatic lesions [8], (3) opportunity offered by PET/ CT to provide a whole body evaluation in a single session (this is particularly relevant in NL because multiple cranial and/or peripheral nerve structures are often involved simultaneously or successively in the course of the disease) [2-4,8]. In lymphomas without NL, PET/ CT response assessment is now usually based on two control PET/ CT studies, an interim study, after a few cycles of chemotherapy, and a PET/CT study following completion of treatment [15]. In NL, insidious relapses and/or progression to other neural structures have been described during the course of initially effective chemo- and/ or radiotherapy [2-4,6,7]. Additional interim PET/CT studies could therefore be mandatory to allow prompt therapeutic adaptations.
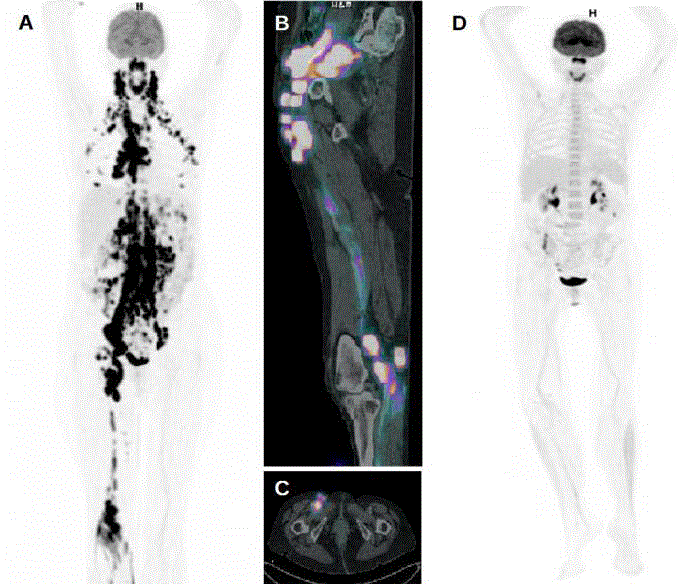
Figure 1A:
Figure 1A:
Baseline 18-FDG-PET whole body scan shows countless
markedly hypermetabolic lymph nodes at all supra- and infra- diaphragmatic
nodal sites, namely cervical, thoracic, abdominal and pelvic sites, as well
as increased FDG uptake within the neurovascular bundle of the right lower
limb, with SUV values ranging between 8 and 20. There are also numerous
intensely FDG-avid nodules in both mammary glands. B. 18-FDG-PET/CT
coronal view of the right pelvis and lower limb shows significant FDG uptake
of the sciatic nerve, extending from its proximal portion in the upper thigh
to its terminal branches, the tibial and fibular nerves divisions, and multiple
FDG-avid pelvic adenopathies. One of them (C, axial view) was biopsied
for non invasive histological confirmation of lymphoma and NL. D. 18-FDGPET
whole body scan performed for early response evaluation after three
cycles of R-CHOP, demonstrates widespread significant regression of the
previously FDG-avid lesions.
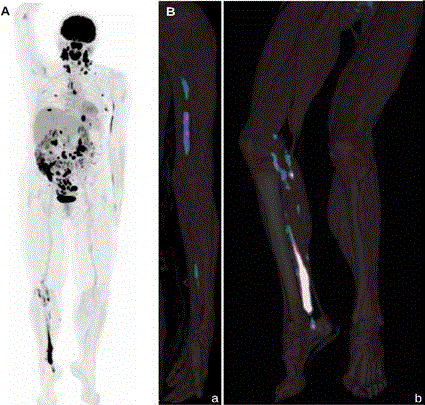
Figure 2A and B:
Figure 2A and B:
18-FDG-PET whole body scan performed six weeks after
the last course of R-CHOP shows widespread tumoral relapse in numerous
supra- and infradiaphragmatic lymph nodes and multiple extranodal sites,
namely left cubital and right sciatic nerves, spinal canal at L1 level, mammary
glands, base of the tongue, nasopharynx, stomach and intestinal loops. B.
18-FDG-PET/CT 3D views of left upper limb (a) and right lower limb (b) show
two different patterns of abnormal 18-FDG uptake in NL, a linear pattern,
with skip lesions, in left ulnar nerve, and a plexiform pattern in right sciatic
nerve divisions.
References
- Denays R, Baudrez V, Abouhamad P, Derème T, Milbouw G, Hermanne JP. Plexiform neurolymphomatosis. Rev Neurol (Paris). 2016; 172: 328-330.
- Grisariu S, Avni B, Batchelor BM, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010; 115: 5005-5011.
- Baehring JM, Batchelor TT. Diagnosis and management of neurolymphomatosis. Cancer J. 2012; 18: 463-468.
- Kamiya-Matsuoka C, Shroff S Gildersleeve K, Hormozdi J, Manning JT, Woodman KH. Neurolymphomatosis: a case series of clinical manifestations, treatments and outcomes. J Neurol Sci. 2014; 343: 144-148.
- Salm LP, Van der Hiel B, Stokkel MP. Increasing importance of 18F-FDG PET in the diagnosis of neurolymphomatosis. Nucl Med Commun. 2012; 33: 907-916.
- Trojan A, Jermann M, Taverna C, Hany TF. Fusion PET-CT imaging of neurolymphomatosis. Ann Oncol. 2002; 13: 802-805.
- Choi YJ, Shin JA, Kim YH, Cha SJ, Cho JY, Kang SH, et al. Neurolymphomatosis of Brachial Plexus in Patients with Non-Hodgkin's Lymphoma. Case Rep Oncol Med. 2013; 2013: 492329.
- Kinoshita H, Yamakado H, Kitano T, Kitamura A, Yamashita H, Miyamoto M, et al. Diagnostic utility of FDG-PET in neurolymphomatosis: report of five cases. J Neurol. 2016; 263: 1719-1726.
- Shree R, Goyal MK, Modi M, Gaspar BL, Radotra BD, Ahuja CK, et al. The Diagnostic Dilemma of Neurolymphomatosis. J Clin Neurol. 2016; 12: 274-281.
- Lin M, Kilanowska J, Taper J, Chu J. Neurolymphomatosis--diagnosis and assessment of treatment response by FDG PET-CT. Hematol Oncol. 2008; 26:43-45.
- Gykiere P, Jans L, Degrieck B, Goethals I. Neurolymphomatosis on 18F-FDG PET/CT: Diagnosis and Therapy Response. Clin Nucl Med. 2016; 41: 142-143.
- Bruce D, Eagleton H2, Subesinghe M3. Diagnostic and response assessment FDG PET-CT in neurolymphomatosis. Clin Case Rep. 2016; 4: 1172-1174.
- Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome in patients with aggressive CD20+B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28: 2373-2380.
- Salles G, de Jong D, Xie W, Rosenwald A, Chhanabhai M, Gaulard P, et al. Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: a study from the Lunenburg Lymphoma Biomarker Consortium. Blood. 2011; 117: 7070-7078.
- Tirumani SH, LaCasce AS, Jacene HA. Role of 2-Deoxy-2-[18F]-fluoro-d-glucose-PET/Computed Tomography in Lymphoma. PET Clin. 2015; 10: 207-225.