Short Communication
Muscle Remodelling on the Basis of Extracellular Matrix Seeded with Cells
Stanislav Filip*
Department of Oncology, Charles University, Czechia
*Corresponding author: Stanislav Filip, Department of Oncology, Charles University, Czechia
Published: 31 May, 2017
Cite this article as: Filip S. Muscle Remodelling on the
Basis of Extracellular Matrix Seeded
with Cells. Clin Oncol. 2017; 2: 1301.
Short Communication
Components of Extracellular Matrix (ECM) incl. basal laminas, connective tissue fibers,
multiadhesive glycoproteins, proteoglycans play a key role in maintaining the structural integrity of
tissues, organs and organisms. In addition to a supportive function, ECM allows cell adhesion and
regulates cell behaviour through binding cell membrane receptors (e.g. integrins), accumulating
bioactive growth factors and altering local tissue stiffness [1,2]. Assembly of ECM components in
mammalian organs is highly ordered and currently available artificial matrices and constructs do
not correspond to complexity of biological systems. Decellularization procedure allows to obtain
a biostroma while retaining organ microstructure and original composition of structural and
bioactive molecules that support cell attachment and organisation. Although acellular scaffolds are
immunologically inert and maintain organ shape and size, after transplantation into the body they
support regeneration only to a certain extent because of the lack of viable cells. On the other hand,
grafts coated with living cells improved their integration into the recipient tissues. Recently several
groups established a proof of concept for utilization of decellularized bioscaffold reseeded with cells
in reengineering of complex bioartificial organs like the heart [3], lung [4] or liver [5]. Such organs
are transplantable and functional as demonstrated by Uygun et al. 2010 in liver grafts or by [6] in
veins.
Skeletal muscles lost in severe injuries may be replaced by autografts in reconstruction surgery;
unfortunately, the availability of a tissue for autografts is severily limited. This problem may be
overcome by construction of artificial muscles prepared after colonization of decellularized muscles
with suitable cells. In the proposed project we plan to exploit our experience gained over years in the
stem cell research. We anticipate the recellularized muscle grafts share several common features with
isotransplants of entire muscles (e.g. of extensor digitorum muscle), which show an excellent muscle
reconstitution after transplantation in recipients [7]. In addition to degeneration of myofibres, other
structures of muscle organ incl. exteroreceptors are affected by denervation and ischaemia resulting
from muscle avascularisation. Nevertheless, basal laminas and satellite cells are left intact in the
is transplanted tissue and they initiate my genesis accompanied with vascularisation, innervation
and renewal of muscle sensory reception incl. Formation of muscle spindles as documented by our
manuscripts [7].
Earlier studies exploited tube-like components of an acellular muscle as a scaffold promoting
axonal regrowth after spinal cord hemidissection, to bridge a gap in the sciatic nerve defects or
to fill the missing muscle tissue in defects created in experimental lesions [1,7,8]. Several reports
described protocols for gaining acellular muscle grafts (e.g., [9-11]. Reseeding acellular muscle grafts
with myoblasts gave rise to engineered muscle constructs with histomorphologic characteristics
that resembled native muscle, which under in vitro conditions were capable of generating
contractile force [3,9]. However, evaluation of grafts reoccupied with myogenic and non-myogenic
cells after implantation in animal models has not yet been performed. Suitable cells for acellular
muscle graft recellularization involve myogenic cells as well as non-myogenic cells that participate
in reconstitution of a tendon, muscle stroma and graft revascularization. The principal cells
responsible for muscle regeneration are satellite cells located at the surface of muscle fibres just
under the basal lamina. A satellite cell is frequently considered to be a progenitor cell due to its
unipotency. The engraftment capacity of isolated satellite cells into injured skeletal muscle is poor
[10] and cells from other sources, e.g. mesoangioblasts [11-14]. Engraft into musle fibres with a
greater efficacy. However, population of satellite cells is highly heterogenous; some are derived
from dermatomyotomes, the others from prechordal mesoderm and few possible other sources. A
minor subpopulation of satellite cells may include myogenic stem cells [14]. Isolated a rare subset
of muscle Side Population (SP) cells characterized by co-expression of ABCG2, Syndecan4 and
Pax7 which occupied satellite cell niche and exhibited more robust
stem cell potential than previously characterized muscle progenitor
cells; after transplantation these were capable of repopulating 75% of
mature myofibers.
Despite muscle-derived cells, another promising candidate for
muscle graft reseeding can be found in the bone marrow-derived
cells. Not only the bone marrow cells contribute to reconstitution of
the stroma in many organs of irradiated animals (our unpublished
results) and tissue revascularization [15,16], they also contribute to
my genesis [17].
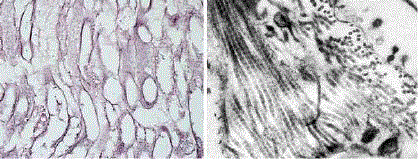
Figure 1
Figure 1
A scaffold of decellularized mouse abdominal muscle we
prepared by immersion in 1% SDS and 0.02% EDTA for 32 hrs. Gomori
impregnation confirms preservation of ECM muscle microstructure of tiny
reticular fibres and absence of sarcoplasm and cell nuclei from myofibers
and endomysium (left). Although a transmission electron microscopy reveals
submicroscopic defects caused by osmotic shock and detergents such as
partial disintegration of basal laminas and interstitial edema it also confirms a
good preservation of collagen fibrils with their characteristic periodic striation
and their organization in bundles (right).
Project Objectives
The main aim of the project is to reconstruct the skeletal muscle organ using ECM scaffold. Since acellular biological matrix can be used only as a passive element (e.g. bridge) that is colonized from outside by adjacent recipient cells (that lack any navigation to enter the graft) we plan to colonize the graft with donor-derived cells immediately prior graft implantation. We hypothesize the combination of myogenic and non-myogenic cells can be optimal for stimulating ingrowth of recipient cells responsible for graft vascularization, innervation and infiltration with inflammatory cells (that initiate the tissue repair). The resulting interaction between recellularized scaffold and recipient cells will support remodelling of the scaffolds microenvironment through partial disruption of basal laminas, local change in ECM composition and reduced stiffness which are critical prerequisites for successful muscle regeneration.
The Main Aim will be Reached Through who Following Principal Activities
Preparation of acellular ECM from the muscle
Muscle injury
In vitro analysis
In vivo analysis
Research Plan
Preparation of acellular ECM from the intact skeletal
muscle tissue
Rationale: The acellular bioscaffold preserves internal microscopic
structure of the muscle that will provide navigation clues to facilitate
entrance and 3D organization of cells participating in the scaffold
reoccupation.
Method
Tissue scaffolding will be prepared from decellularization of the
anterior tibial m. and external digitorum longus m. of C57BL/6J
and GFP (3 month-old) mice using different protocols. GFP mice
are the strain C57BL/6-Tg(CAG-EGFP)C14-Y01-FM131Osb mice
(Riken) – cooperating laboratories (Dept. Histology and Embryology
and Dept. Biochemistry) are included in the register of GMO users
(see http://www.mzp.cz/www/env-gmo.nsf/e7244034d91e3db0c125
6e7e003a0828/2c41eb4ec5cda35dc1256fe9004176d4?OpenDocume
nt). Scaffolds are prepared by combination of initial osmotic shock
followed by immersion with ionic or zwitterionic detergents that
are then replaced with prolonged washes with PBS with the aim to
remove cell cytoplasmic components and preserve ECM proteins and
glycosaminoglycans (in our previous experiments better results for
muscle acellularization were reached with immersion technique than
with whole-body perfusion decellularization.
A methodical part of the study plans to look the way for optimal
storage of decellularized matrix with the use of physical and chemical
approaches (cryopreservation, solutions for keeping the graft in
aseptic conditions in the fridge or at room temperature, mild fixation
with paraformaldehyde/glutaraldehyde). In addition to processing
the entire muscle for decellularization, we also plan to decellularize
small muscle units (from tissue cubes 1-2 mm in length to smaller
fascicles). Processing small muscle pieces consisting of few myofibers
should offer several advantages: we expect, their decellularization
occurs faster with lower deteriorative effects on ECM and small
units become more easily colonized by the cells. Because the basic
skeletal muscle architecture is formed by multiple parallelly arranged
myofibers, the parallelly arranged subunits could be used to fill in
the large gaps in the traumatized muscle. Re-assembly of the small
muscle units will be studied in vitro, the precise orientation and
arrangement of units with adhered paramagnetic particles will be
achieved with a magnetic field and with avidin to bridge biotinylated
unit tips. Efficacy of the decellularization process will be evaluated
with light, histochemical (Sirius red, Gomori impregnation, alcian
blue, dimethylmethylene blue, biotinalyed hyaluronic acid binding
protein to detect collagen and reticular fibres, proteoglycans and
hyaluronic acid) and fluorescent microscopic methods; quantification
is performed with Image analysis (Image Pro). Transmission electron
microscopy is used to confirm removal of cellular components incl.
chromatin and preservation of ECM structure; Western blotting is
used to assess the presence of biogenic ECM molecules incl. decorin,
biglycan, fibronectin, laminin and heparin sulphate proteoglycans in
the grafts and to confirm a removal of such cellular proteins like actin.
An advantage of preparing scaffolds from transgenic mice with an
enhanced GFP expressed under the control of a chicken beta-actin
promoter is a rapid assessment of decellularization efficacy because a
removal of cellular components correlates well with a decline and loss
in GFP fluorescence.
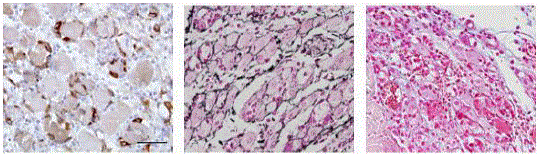
Figure 2
Figure 2
Muscle regeneration on day 4 after CTX injection. Nestin+
myoblasts derived from activated sattelite cells identified by imunoperoxidase
immunohistochemistry (left), reticular fibres delineating myotubes idenitifed
by Gomori impregnation (middle), ECM sulphated mucopolysaccharides by
alcian blue and cell nuclei by Nuclear Fast Red (right).
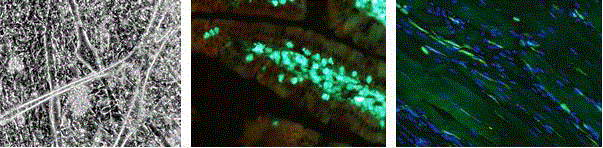
Figure 3
Figure 3
Formation of multinucleated myotubes from embryonic myogenic
cells is facilitated by a decrease in serum concentration and cultivation on
a soft hydrogel (left). Fluorescent identification of GFP+ bone marrow cells
transplanted to irradiated mice; after 70 days the cells participate in stroma
repair (middle). GFP+ bone marrow cells transplanted to animals 28 days
after CTX muscle injury infiltrate the muscle stroma (right).
Muscle Injury Analysis
Rationale: Precise knowledge of events associated with adult
myogenesis incl. their timing (inflammatory reactions, cell activation,
proliferation, differentiation) and characterization of changes during
muscle ECM remodelling (matrix metalloproteinases /MMPs, ECM
glycoproteins deposition etc.) is of critical importance for creating
promyogenic niche that stimulates muscle regeneration.
Method
We plan to exploite the model of adult muscle injury induced
by 100μl 10μM cardiotoxin (CTX) injection into the anterior tibial
muscle of 2-3 moth-old C57Bl6/J or GFP mice. Our previous
findings documented that following a removal of necrotic myofibres
on day 3, the myogenesis occurs after activation of intact satellite
cells left in situ in the close vicinity to necrotic myofibres and
includes both extrafusal and intrafusal myofibres [17]. In addition
to characterization of myogenic cells, an attention will be paid to
expression of MMPs (particularly those involved in satellite cell
activation – MMP9, degradation of basal membrane components -
MMP7 [18], MMP10, degradation of entactin – MMP7, 8, 13) and
expression of ECM glycoproteins (fibronectin, laminin, entactin). The
site of degeneration/regeneration will be analyzed histologically in
defined intervals of time (on day 3, 4, 5, 7, 10 and 21 post injury) using
histochemistry, electron microscopy as well as immunohistochemical
detection of myogenic markers (Myf5, MyoD, myogenin, striated
muscle myosin heavy chains /SM1 and SM2/, intermediate filaments
incl. nestin, vimentin, desmin) to get a detailed knowledge of temporal
processes associated with muscle regeneration, markers of muscle
cell proliferation, activation (integrins β1 [mediate myogenesis, i.e.
heterodimers with α1, α3, α4, α5, α6, α7 α5, αv]; β3 [αvβ3 in myoblasts
and activated satellite cells - [19]; β4 [interstitial cell progenitors -
[20] and ECM glycoproteins.
Injured muscles will also serve as controls to myogenesis in
decellularized and recellularized grafts and for isolation of activated
myogenic cells (see below). Regenerating muscles on day 4-5 from
CTX injury which undergo substantial ECM remodelling with
promyogenic niche (caused e.g. by perisatellite deposition of cellular
fibronectin [21] and other bioactive molecules) will be decellularized
and the resulting scaffolds used for recellularization experiments.
In Vitro Analysis
Rationale: Muscle regeneration requires the coordinated
interaction of multiple cell types. Myogenic cells alone are not able
to reconstruct complex structures in the muscle organ. Therefore we
plan to seed the acellular scaffolds with distinct cell types: either with
myogenic cells alone or non-myogenic cells alone or combination of
both types. The most promising cell populations will be defined from
analysis cell behaviour on acellular scaffold surfaces grown in vitro.
Method
In vitro tests are based on cultivation of cells on 2D acellular
scaffold cryosections attached to the bottom of cultivation dishes
and in 3D scaffolds cultured for 1-2 weeks. When using small
decellularized muscle units, the constructs will be seeded with
cells after the re-assembling; following cell immigration and
differentiation, morphology of such constructs will be compared
to large recelularized scaffolds. Scaffolds will be examined in their
natural stage after their preparation or after modification with MMP7
(to inititate remodelling of basement membranes) or treatment
with fibronectin and Wnt7 (to stimulate satellite cells). As a control,
acellular scaffolds from non-muscle organs, peripheral nerve or liver
will be used.
Myogenic cells used for in vitro tests include C2C12 myoblasts
(an immortal line of mouse skeletal myoblasts originally derived from
satellite cells) and muscle-derived stem cells. The latter population
is obtained from intact or injured muscle using a modified preplate
technique described by [21]. as the cells received from muscle
dissociation with the slowest adhesion to the flasks (after 5-7 serial
transfers). The stem cell population (Sca-1+, CD34+) expand in
vitro in DMEM supplemented with 20% FBS within 10 days and
is maintained in undifferentiated conditions at low cell density
culture. This cells population shows increased capacity to engraft into
myofibres and satellite cell niche [22]. Additional immunomagnetic
separation for β4 integrin or ABCG2/Syndecan4 will be used to yield
a subpopulation of myogenic cells with robust engraftment capacity
[23-25].
Non-myogenic cells will be obtained from the bone marrow
mesenchymal cells or muscle connective tissue fibrobalsts. We
expect non-myogenic cells to participate in formation of muscle
microenvironment, coverings and tendon etc. From the bone marrow,
all differentiated blood elements are removed with lineage cell
depletion kit; the resulting suspension enriched for progenitor and
stem cells will be used for graft recellularization. Alternatively positive
immunoselection will be performed to collect cells expressing the
required markers (CD117/c-kit, Sca-1, CD133). Muscle connective
tissue cells are obtained from a preplate technique described above as
the cells received from muscle dissociation with increased adhesion
to the flasks (after 2-4 serial transfers). Cells that reveal myogenic
potential in differentiation culture are eliminated with CTX.
Isolated cells are characterized in our labs with measurement
of cell kinetics, viability and immunophenotypization
(immunofluorescence, flow cytometry using the flow cytometer
CyAN-ADP (DakoCytomation) and with the expression of cellspecific
markers (e.g. myogenic regulatory factors MyoD, Myf5,
myogenin). Identification of primary cillia (using anti-acetylated
α tubulin or glutamylated tubulin immunofluorescence) will be
examined in cells because these sensory structures are associated with
multiple signalling pathways; Wnt and Shh signalling in myogenic
cells (e.g. [26] offer possibility for modulation of stem/progenitor cell
differentiation toward required myogenic phenotypes.
The acellular grafts are recellularized by multiple injections of
103 to 104 donor cells (depending on the graft size) in different sites
of the graft under the visual control with a stereomicroscope and
cultured in vitro. The cells with best potential to infiltrate the scaffold
and participate in myogenesis are to be utilized for recellularization.
3D scaffolds seeded with cells are analyzed for biocompatibility,
adhesion, cell surival, proliferation, migration and differentiation
depending on number, and type of seeded cells and time period using
histological examination and compared to regeneration induced by
CTX. Presence and length of primary cillia will be correlated with the
cell capacity to colonize acellular grafts.
In Vivo Analysis
Rationale
A complete tissue remodelling requires participation of recipient
and its inflammatory cells, vascularisation, innervation etc. For that
reason acellular and recellularized bioscaffolds will be implanted
into the recipient muscle; rate and efficacy of muscle remodelling
will be evaluated histologically. We expect recellularized scaffolds
to show higher rate of remodelling because cells inside the scaffold
become hypoxic after transplantation and trigger signalling through
proangiogenic factors to attract blood capillary ingrowth. Graft
vascularization is crucial not only for substituting cells with oxygen
and nutrients to support cell survival and growth but it allows entry
and spreading of other mesenchymal elements via perivascular spaces
and involvement of new circulating stem/progenitor cells.
Method: Small scaffolds will be placed in a cavity created within
the anterior tibial muscle of anaesthetised animales and covered
with sutured fascia. For recellularization, GFP+ myogenic and/
or non-myogenic cells will be used to allow identification of cells
after transplantation into histocompatible GFP- C57Bl6/J mice.
Implantation of decellularized and recellularized muscles grafts is
performed in the same way as isogenous transplantations described
by us earlier [27]. Proximal tendon of the graft is ligated, pulled
distoproximally through the exposed host extensor digitorum
longus muscle then sutured at the proximal pole of the host muscle
and the distal tendon of the graft is connected to the distal pole of
the host muscle. After transplantation, the cut tendon of the host
tibialis anterior muscle is sutured to the site of its insertion and then
the skin is sutured as well. After 24 hrs mice are treated with 33 mg
bromodeoxyuridine i.p. injection and after 72 hrs with iododeoxyridine
to allow sequential staining of different subpopulations of activated
cells. Experimental animals will be divided into four groups (scaffolds
acellular, recellularized with myogenic cells only, non-myogenic cells
only and both populations) at a minimum of six animals each (for
each survival interval).
Muscles are explanted under a dissecting stereomicroscope
(after 4, 7, 21 days) and examined under the UV lamp. Sections
will be cut on a cryostat and examined under a fluorescent
microscope. Precise morphological examination is performed in
paraffin-embedded sections (GFP+ cells are detected with anti-
GFP immunohistochemistry). For identification of phenotype of
transplanted cells, co-expression of cell-specific antigens (myogenic
markers), bromodeoxyuridine and laminin, the immunofluorescent
staining will be performed. Image analysis (Image Pro) is used
to evaluate the extent of cell engraftment and for estimation of
muscle regeneration by calculating number of myotubes with
centrally located nuclei. Graft innervation is assessed from S100 or
neurofilament immunohistochemistry; graft vascularization from
PECAM or nestin immunostaining or after Indian ink perfusion of
bloood vessels. Transmission electron microscopy: Characterization
of graft scaffolding, initial steps of graft repair, examination of nerve
fibres (e.g. their myelination) and blood vessel wall maturation (e.g.
presence of pericytes) is done with TEM in Durcupan-epoxy resins
after osmification [27]. Quantitative PCR is used as an independent
method to verify the presence of GFP cells in DNA of the examined
tissue containing exogenous cells using modification of the procedure
described by us [28]. Functional tests of mice injured, transplanted or
implanted with bioscaffolds will include Rotarod tests and Foot print
analysis. Data analysis will be performed by appropriate statistical
methods using software Statistica v. 9.0 (StatSoft) and GrapPad Prism
v. 5.01 (GraphPad).
References
- Arai T, Kanje M, Lundborg G, Sondell M, Liu XL, Dahlin LB. Axonal outgowth in muscle grafts made acellular by chemical extraction. Rest Neurol Neurosci. 2000; 17: 165-174.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origine of endothelial progenitor cells responsible for postnatal vasculogenesis in physological and pathological neovascularisation. Circ Res. 1999; 85: 221-228.
- Borschel GH, Dennis RG, Kuzon WM Jr. Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast Rec Surg. 2004; 113: 595-602.
- Calve S, Simon HG. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. Faseb J. 2012; 26: 1-8.
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008; 134: 37-47.
- Cízková D, Soukup T, Mokrý J. Nestin expression reflects formation, revascularization and reinnervation of new myofibers in regenerating rat hind limb skeletal muscles, Cells Tissues Organs. 2009; 189: 338-347.
- Cízková D, Soukup T, Mokrý J. Expression of nestin, desmin and vimentin in intact and regenerating muscle spindles of rat hind limb skeletal muscles. Histochem Cell Biol. 2009; 131: 197-206.
- Danoviz ME, Yablonka-Reuveni Z. Skeletal muscle satellite cells: Background and methods for isolation and analysis in a primary culture system. J Cell Biol. 2011; 195: 147-163.
- Doyle MJ, Zhou S, Tanaka KK, Pisconti A, Farina NH, Sorrentino BP, et al. Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J Cell Biol. 2011; 195: 147-163.
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G. et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998; 279: 1528-1530.
- Filip S, Vávrová J, Vokurková D, Bláha M, Vanásek J. Myeloid differentiation and maturation of SCF+IL-3+IL-11 expanded AC133+/CD34+ cells selected from high-risk breast cancer patients. Neoplasma. 2000; 47: 73-80.
- Fuksa L, Brcakova E, Kolouchova G, Hirsova P, Hroch M, Cermanova J, et al. Dexamethasone reduces methotrexate biliary elimination and potentiates its hepatotoxicity in rats. Toxicology. 2010; 267: 165-171.
- Gillies AR, Smith LR, Lieber RL, Varghese S. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Eng. 2011; 17: 383-389.
- Karalaki M, Fili S, Philippou A, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo. 2009; 23: 779-796.
- Li Y, Pan H, Huard J. Isolating stem cells from soft musculoskeletal tissues. J Vis Exp. 2010; 41.
- Li P, Xu S. Transplantation of autologous muscle basal lamina and use of nerve growth factor to repair spinal cord transection in dogs: an histological observation. Chin J Spine Spinal Cord. 2000; 10: 220-223.
- Liadaki K, Casar JC, Wessen M, Luth ES, Jun S, Gussoni E, et al. Beta-4 integrin marks interstitial myogenic progenitor cells in adult murine skeletal muscle. J Histochem Cytochem. 2012; 60: 31-44.
- Liu H, Niu A, Chen SE, Li YP. Beta-3integrin mediates satellite cell differentiation in regenerating mouse muscle. Faseb J. 2001; 25: 1914-1921.
- Madhala-Levy D. Cooperation between Shh anf IGF-I in promoting myogenic proliferation and differentiation via the MAPK/ERK and PI3K/Akt pathways requires Smo aktivity. J Cell Physiol. 2012; 227: 1455-1464.
- Mokrý J. Microstructure of nonpassaged spheroids formed by EGF-responsive neural precursor cells in vitro. Elec J Pathol Histol. 1999.
- Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet. 2012; 380: 230-237.
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: Using nature's platform to engineer a bioartificial heart. Nat Med. 2008; 14: 213-221.
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010; 329: 538-541.
- Qing Q1, Qin T. [Optimal method for rat skeletal muscle decellularization]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009; 23: 836-839.
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006; 444: 574-579.
- Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. et al. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009; 4: 217-225.
- Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010; 16: 814-820.
- Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010; 31: 7475-7484.