Research Article
MR Guided Focused Ultrasound for the Palliation of Bone Metastases: Clinical Procedures and Outcome
Lili Chen1*, Joshua Meyer1, Gary Freedman2, Andre Konski2 and C-M Ma1
1Department of Radiation Oncology, Fox Chase Cancer Center, Philadelphia, USA
2Department of Radiation Oncology, Perelman Center for Advanced Medicine, Philadelphia, USA
3Department of Radiation Oncology, The Chester County Hospital, West Chester, USA
*Corresponding author: Lili Chen, Department of Radiation Oncology, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA
Published: 15 May, 2017
Cite this article as: Chen L, Meyer J, Freedman G, Konski
A, Ma C-M. MR Guided Focused
Ultrasound for the Palliation of Bone
Metastases: Clinical Procedures and
Outcome. Clin Oncol. 2017; 2: 1287.
Abstract
Purpose: The purpose of this paper is to report our initial clinical experience with MR Guided
Focused Ultrasound (MRgFUS) for the palliative treatment of bone metastases with a focus on
patient treatment workflow and Quality Assurance (QA) procedures to ensure the safe and effective
operation of MRgFUS.
Methods: In addition to the monthly, annual QA and Preventive Maintenance (PM), pre-treatment
machine QA was performed one week before and on the day prior to patient treatment to test
the machine hardware, imaging and treatment software and patient safety devices. The geometrical
ultrasound focal spot between the planning focal spot and treatment focal spot was registered with
an acoustic phantom using MR thermometry. The QA procedures were verified through patient
treatments. Seven patients (recruited in the multicenter Phase III clinical trial, BM004) with scapula,
humeral head, sacrum, ilium, pubic ramus and acetabular bone metastases were treated using a
FDA approved clinical focused ultrasound system under MR guidance. Patients were treated with
a frequency of 1 MHz; acoustic power of 32W ± 4.0W to 96W ± 11W with a duration of 20-30
seconds for each sonication resulting in an energy of 628J ± 78J to1859J ± 338J. MR phase images
were used to monitor the temperature changes at focal spots in real-time. Based on the temperature
feedback, the acoustic power can be adjusted to reach designed temperatures (≥600C) for individual
sonications. The effectiveness of the treatment was evaluated by pain score using the visual analog
scale (0-10VAS).
Results: Our data showed that all seven patients tolerated the MRgFUS treatment well. No skin
toxicity or other complications were observed during or after treatment. The patient pain rating was
significantly reduced from 8.0 ± 1.1 before treatment to 4.7 ± 3.0, 3.0 ± 1.5, 3.2 ± 2.8 and 3.4 ± 1.5 at
one day, one month, two months and three months after treatment, respectively.
Conclusions: Our clinical data suggests that with appropriate treatment and QA procedures,
MRgFUS is a safe and effective treatment modality for the noninvasive palliation of bone metastases.
Keywords: Quality assurance; MR guided focused ultrasound; Bone metastases
Introduction
High Intensity Focused Ultrasound (HIFU or FUS) is a completely non-invasive treatment
modality. This technique has long been known to offer “trackless lesioning” and has been identified
as an “ideal surgical tool” for many years but only in the past decade with high quality methods
using medical imaging, has it become a practical option in clinical treatment [1]. This high quality
image technique not only provides accurate target delineation and ultrasound beam placement but
also enables real-time assessment of treatment effects during the treatment procedure.
FUS has been developed for tissue ablation using the continuous ultrasound mode to treat both
benign and malignant diseases, such as patients with uterine fibroids, Benign Prostatic Hyperplasia
(BPH), prostate cancer, renal tumors, hepatic tumors, breast cancer, brain tumors and for palliative
treatment of bone metastases to relieve pain, etc. [2-11]. Current imaging modalities being used for
treatment guidance include ultrasound and MR imaging [12,13].
FUS has also been investigated to enhance local drug (or enhancement agent) delivery using
the pulsed mode in animal models for chemotherapy, gene therapy, immunotherapy and radiation
therapy [12,14-20].
A clinical phase III study (BM004 ExAblate) to evaluate the
effectiveness and safety of ExAblate treatment of metastatic bone
tumors for the palliation of pain in patients who are not candidates
for radiation therapy has been completed successfully [21]. The goal
of the treatment is to relieve the pain from the bone metastases and
the principle of the treatment is to ablate the adjacent periosteum of
the bone which is the sensory origin of the pain. Based on the success
of this clinical trial, MR guided high intensity focused ultrasound
(MRgFUS) for palliative treatment of bone metastases was approved
for routine clinical practice by the United States Food and Drug
Administration (FDA) in October, 2012.
Since it is a new treatment technique, detailed clinical Quality
Assurance (QA) procedures and treatment workflow are not available
in the literature to our knowledge. Appropriate QA procedures play
an important role in the success of MRgFUS treatment for both safety
and effectiveness. The purpose of this paper is to describe our clinical
experience with MRgFUS for the palliation of bone metastases with
a focus on treatment workflow and QA procedures through patient
treatments based on our 7 patients recruited for the BM004 clinical
trial and 15 patients treated after FDA approval for clinical treatment
using a FDA approved clinical HIFU treatment system.
Methods and Materials
MRgFUS treatment system
The clinical focused ultrasound treatment system (ExAblate
2000 - Insightec) was used in this study. The HIFU treatment system
is approved by FDA for the treatment of uterus fibroids and bone
metastases clinically. The MR (1.5T) scanner (GE medical system)
was used for MR guidance in target delineation, treatment planning,
ultrasound beam placement, and temperature measurement during
treatment using MR thermometry. The main components of the
system include the patient treatment table, operator console and the
equipment cabinet.
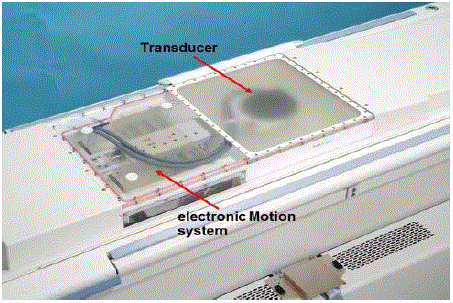
patient treatment table, ultrasound transducer and positioning
system: The patient treatment table is a modified SIGNA MRI Table.
It is detachable and can be docked to a GE 1.5T/3T MR scanner in
the same way that the standard MR table docks: it is connected with
a single quick-connect socket. A phased array transducer with 208
elements is housed in a sealed degassed water bath in the patient
treatment table and is connected to an electronic motion system
controlled by a computer (Figure 1). The transducer can be moved
in X Y directions (but cannot be moved up and down). It can also
be tilted relative to its neutral position, parallel with the Table. The
focal length of the transducer is 16cm (for each element, not using
electronic steering). However, since the transducer is a phased array
transducer, the phases of each element can be changed to create a new
focus and therefore steer the focal length range from 6cm to 22cm
away from the transducer. The focal size can be varied depending on
the size and depth of the focal volume to be treated, ranging from
1mm diameter x 8mm length to 10mm diameter x 45mm length.
The operating frequency is around 1MHz ranging from 0.95MHz to
1.35MHz. For bone treatment, the frequency used is typically 1MHz.
The equipment cabinet is located adjacent to the MR control room.
It contains a main power switch along with electrical components.
Operator console: The console is located in the control room next
to the MR workstation. It includes a flat panel display, keyboard, and
mouse and stop-sonication button. It controls the entire treatment
process by the physician.
The treatment console is used for 1) image fusion, 2) target
delineation, 3) treatment planning, 4) controlling both the therapeutic
table (transducer motion and energy delivery), and the MR scanner,
5) assessment of the treatment by analyzing and displaying each
energy delivery outcome in real-time including thermal feedback
using MR thermometry and for thermal dose with color overlay on
the treated spots.
Safety devices: Safety devices include a sonication lamp, which
is installed in a prominent position on the MRI scanner and lights
up during treatment sonication, and three stop-sonication switches,
which instantaneously stop the delivery of ultrasound energy to the
patient for emergency. One is held by the patient who is instructed to
squeeze it in case of sudden discomfort or for an emergency; another
is mounted on the scanner for use by staff in the treatment room;
and the third switch is installed in the FUS operator console in the
control room.
Patient treatment protocol
Study Protocol BM004, a randomized phase III trial, was a
multicenter, single-blinded, randomized study to evaluate the efficacy
and safety of treatment for metastatic bone tumors using the ExAblate
for the palliation of pain in patients who have failed radiation or who
were not candidates for or refused radiation therapy [21]. Patients
randomized in the sham group received no acoustic power during
the treatment. Three weeks after the treatment the patient was entered
in the treatment group. A total of 148 patients were recruited for the
study in the U.S. and internationally.
Safety was evaluated by assessing the incidence and severity of
device-related complications from the first treatment day through the
3-month post–treatment time point. Effectiveness of the treatment
was evaluated based on treated patients who experienced at least a
2-point improvement from baseline on a numeric rating scale NRS
(0-10) at the treated site without an increase in medications.
Treatment team
The treatment team at Fox Chase Cancer Center (FCCC) for this
study included one radiation oncologist, one project coordinator; two
registered nurses (advanced cardiovascular life support certified),
one medical physicist and one board certified MR technologist.
Each person performed a specific role in the study. The radiation
oncologist was responsible for recruitment of patients, performing
treatment planning, patient treatment and the follow-ups after
treatment. The project coordinator was responsible for coordinating
patient treatment with the treatment team, patient and study sponsor (Insightec) and also for maintaining the treatment records. Two
registered nurses were responsible for working with the operating
physician to provide an appropriate level of sedation and continuous
monitoring of vital signs (pulse, blood pressure, oxygen saturation)
with MRI compatible equipment. One nurse remained with the
patient in the treatment room for the entire treatment time. The
medical physicist was responsible for treatment QA (machine
calibrations including the hardware, software and all safety devices).
The MR technologist worked with the physicist for patient treatment
setup and was also responsible for performing MR images before,
during and after treatment.
Machine calibrations
Daily QA (DQA): In order to allow the vendor to have adequate
time to correct potential problems from QA without delaying the
patient’s scheduled treatment, we performed DQA at least one week
prior to the patient’s treatment.
Mechanical check: 1) To reboot the MR scanner, 2) to dock the
HIFU treatment table to the MR scanner and check the mechanical
movements for table in and out; up and down positions, 3) attach
the coupler cable to the treatment table, 4) turn on the HIFU work
station and log into the computer, 5) connect the pelvic coil to the MR
scanner, and 6) set up the landmark for MR scan.
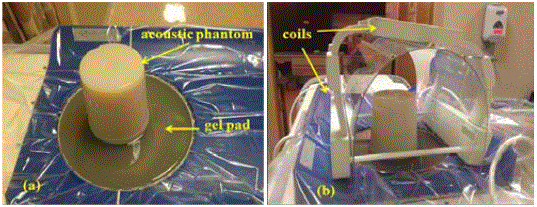
QA phantom set up: A gel pad was placed on the treatment table
in line with the transducer. Degassed water was used for the interface
between the treatment table and the gel pad for the acoustic coupling.
Care was taken to eliminate air bubbles between the interfaces. The
gel pad is used not only for supporting the acoustic phantom but also
as a medium for ultrasound transmission. The acoustic phantom was
placed on top of the gel pad (Figure 2). The phantom was manufactured
by ATS Labs, Inc. (Bridgeport, CT) with acoustic properties similar to
those of human soft tissue (the attenuation coefficient = 0.503 dB cm-1
MHz-1; speed of sound = 1538 MPS; estimated specific heat = 2.684
cal/g) except for without the property of thermal conductivity due to
lack of blood flow. The acoustic phantom is not used for patient tissue
calibration. It is used for mechanical calibration and software for the
end to end test. The phantom was used for identifying the geometrical
treatment focal spot by temperature elevation during the sonication.
The effective treatment focal spot was registered with the template
focal spot in the planning software (see below). The verification of
the focal spot position in patients is achieved using low-energy
subtherapeutic sonications and MR thermometry.
End-to-end test: Successful completion of the end-to-end test
indicated that all parts including the mechanical hardware, MR
scanner, MR coils, and treatment software have passed QA. For the
end-to-end test, a 3-plane localization of MR sequence was performed.
The images were carefully checked for any gas bubbles between the
interfaces. The images were loaded to the FUS workstation. The
position of the transducer from the images was calibrated to the
contour of the transducer from the treatment software. The effective
treatment focal spot was registered in 3 imaging planes using MR
thermometry. Finally, all of the safety devices (described as above)
were tested.
Preventive maintenance (PM): A Preventive Maintenance (PM)
program was developed for both quality and safety control. PM is
performed by a trained engineer from the vendor. The quality control
checks may include items such as alignment light, coils, patch update,
large volume shim, eddy current class, coherent noise, SNR and
spike noise etc. while the safety control checks may include oxygen
monitor operation, patient blower & filter, patient table, pneumatic
patient alert system and magnet rundown unit. A PM procedure is
performed every 6 months in the Radiation Oncology Department
of FCCC.
Patient preparation and treatment setup
On the day of the treatment, each patient reported to the clinic
in the Department of Radiation Oncology 2 hours before treatment
for preparation, including removal of the skin hair (if any) in the
treatment area, placement of the intravenous line and Foley catheter
and application of anti-thrombotic compression devices. The patient
was also sent to Interventional Radiology for a periosteal injection
consisting of bupivacaine and epinephrine into the region of the
desired treatment target under fluoroscopic imaging guidance.
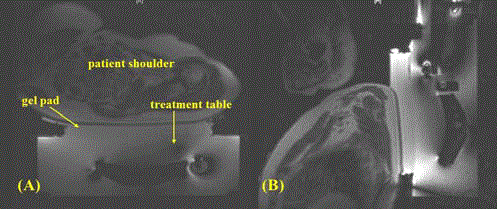
A pelvic MR coil (23cm circular hollow in the center) was placed
and secured on the treatment Table. A plastic sheet was placed on the
Table. The interface between the treatment Table and the plastic sheet
was coupled using acoustic gel and degassed water. The edges of the
plastic sheet were secured on the coil frame using medical plastic tape
so that it appeared comparable to a large plastic bowl. A 2cm thick
gel pad was placed on the plastic sheet with degassed water for an
acoustic coupling. The degassed water was filled to a few mm above
the gel pad.
The ideal position for patient setup is to have the treatment target
in line with the center of the transducer. In order to save time for
patient setup, a temporary skin marker indicating the position relative
to the target was created using the guidance of diagnostic CT images.
The patient was laid on the gel pad in contact with the degassed
water. The skin marker related to the treatment site was positioned
in line with the center of the transducer. Initially, a localization scan
(axial, sagittal and coronal) was performed. From the MR images, the
interfaces between the treatment table and gel pad, the gel pad and
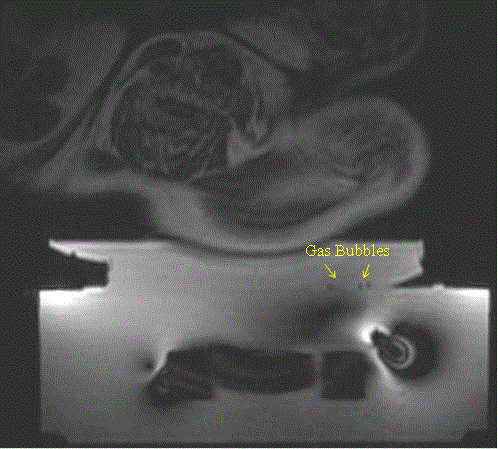
patient’s skin were carefully checked for gas bubbles (Figure 3).
Treatment procedures
The patient was treated under conscious sedation. A registered
nurse was with the patient in the MR room for the entire treatment
procedure working with the physician to provide an appropriate
level of sedation. Continuous monitoring of vital signs (pulse, blood
pressure, and oxygen saturation) was implemented by the nurse with
MRI compatible equipment.
Prior to treatment, patient CT images were loaded onto the
MR console. The patient was instructed to hold a panic button for
emergency purposes during treatment. Both axial and sagittal T2-
weight MR scans were performed. Both MR and CT images were loaded to the HIFU treatment station. CT and MR fusion was
performed using built-in software. The target outlining and treatment
planning were performed by the treatment physician in real-time.
Patients were treated with a frequency of 1 MHz; acoustic power
of 32W ± 4.0W to 96W ±11W and energy of 628J ± 78J to1859J ±
338J for 20-30 seconds for each sonication. Temperature changes
(in the soft tissues immediately adjacent to the treated bone target)
were monitored in real-time using MR thermometry with the proton
resonant frequency shift method. Based on the temperature feedback,
the acoustic power was adjusted to reach the desired temperature
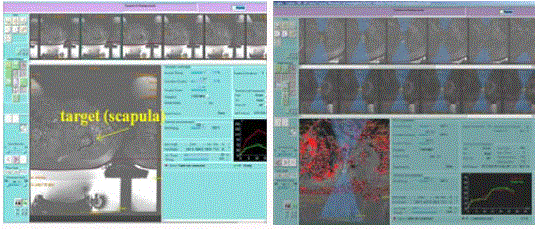
(≥600C) for each individual sonication. Figure 4 demonstrates realtime
MR guidance for FUS treatment. Patients were followed up to 3
months after treatment based on the study protocol.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 4
A screenshot demonstrating (a) real-time treatment planning, and
(b) treatment monitoring with MR thermometry.
Results and Discussion
QA
Pre-treatment QA plays an important role in performing
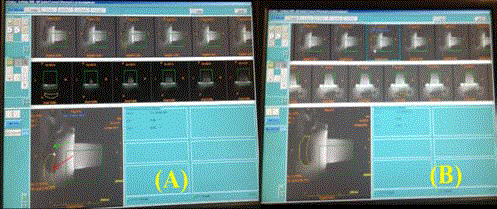
the MRgFUS treatment safely and effectively. Figure 5 shows
the registration of the transducer position between the physical
transducer and the transducer contour from the treatment software
using MR guidance. In our practice, in order to avoid the possibility
of cancellation of patient treatment due to treatment machine
malfunctions, pre-machine QA (a few days before) is necessary. For
example, we found that during the pre-treatment QA for one of the
seven patient treatments, the transducer could not be returned to the
home position; therefore, we informed the vendor without delaying
the patient’s treatment. The “new” QA procedure translated into noncancellation
of treatments.
Figure 6 demonstrates an example of the gas bubbles trapped
between the interfaces, which must be removed before the sonication
treatment. During the patient setup, we carefully removed all gas
bubbles and further checked this through MRI imaging. We believe
that with the clinical treatment protocol (treatment target is >1cm
from the skin surface), skin toxicities should not occur unless gas
bubbles exist between the interfaces of the skin and the gel pad.
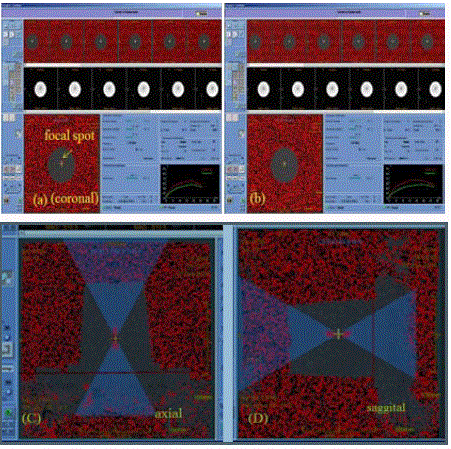
Figure 7 is the registration of the treatment focal spot to the
treatment planning focal spot in 3 dimensions demonstrating realtime
closed loop quality assurance procedure.
Patient treatment parameters and treatment outcomes
The principle of bone palliative treatment is to use FUS to ablate
the adjacent periosteum, which is the sensory origin of the pain.
The benefits of MRgFUS for palliation of pain in bone metastases,
generally include a single treatment session, a non-invasive treatment
procedure, nonionizing therapy, and fast pain relief. Table 1
summarizes the information for seven treated patients including the
primary tumor, treated site, treatment parameters, the total number
of sonications and the temperature reached in the treated target.
On average, the temperatures from MR thermometry in the
target ranged from 620C to 770C with a standard deviation of ± 70C
-120C. All 7 patients tolerated the MRgFUS treatment well including
15 additional patients treated after the system received FDA approval.
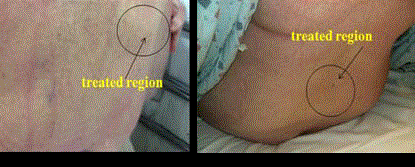
No device-related severe adverse events were recorded for any of
the patients. There were no skin toxicities or other complications
identified. Figure 8 represents a picture taken immediately following
HIFU treatment demonstrating no skin toxicity from the treatments.
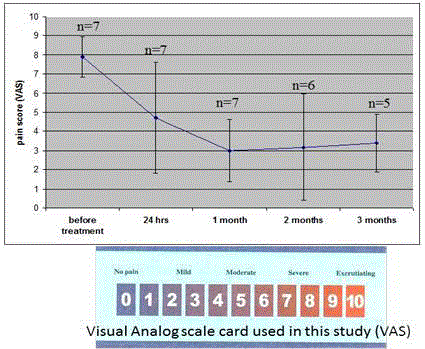
Our data showed that the numerical pain scale was significantly
reduced from 8.0 ± 1.1 before treatment to 4.7 ± 3.0, 3.0 ± 1.5, 3.2 ±
2.8 and 3.4 ± 1.5 at one day, one month, two months and three month
intervals after treatment, respectively (Figure 9). Based on the clinical
trial protocol, responders were defined as a decreased VAS pain score
of 2 points without a 25% increase in narcotic pain medication. The
average VAS pain score from our study was reduced from 8.0 prior
to treatment to 3.4 at 3 months post-treatment (a 4.6 point decrease).
Our results are consistent with those reported by other participating
centers. Liberman “et al”. [7] showed that the average VAS score was
reduced from 5.9 prior to treatment to 1.8 at 3 months post-treatment
(a 4.1 point decrease). Gianfelice “et al”. [4] reported that all patients
showed a progressive decrease in pain in the treated regions. VAS
scores averaged 6.0 before treatment and decreased to 0.5 at 3 months
(decrease in pain score, 92%; P < 0.01) without any adverse events.
Catane “et al”. [21] also reported a prolonged improvement in pain
score and/or reduced analgesic dosage without any severe adverse
events.
Thermal feedback with MR thermometry using the proton
resonant frequency shift method [23] in bone treatment is a challenge
since bone produces weak signals on MRI due to the low water
content of bone cortex. Therefore, the temperature increase inside
the bone tissue cannot be directly measured. The reliable temperature
measurements were obtained from soft tissue adjacent to the treated bone. The treatment system embedded software calculates the
temperature elevation providing temperature mapping on the phase
images, which exhibited some noises inside bone, fatty areas, and
near interfaces between tissues, etc. Therefore, it is a challenge for the
operator to manually select the real heating area accurately by drawing
a polygon. Only the thermal dose [24] inside the drawn polygon will
be collected for treatment reference. However, the outcome of the
treatment is very effective and significant from our clinical experience
and the studies from other institutions, as mentioned above [21].
The significance of this work is not only to report the favorable
clinical outcome of MRgFUS bone palliation that is consistent with
those reported but more importantly to demonstrate practical QA
procedures which is essential for its safe and effective operation.
The QA procedures and the clinical workflow described in this
paper would be of assistance to other institutions in gaining clinical
experience using the same treatment systems.
The limitation of this study was a small patient population.
However, we also mentioned an additional 15 patients treated after
the system received FDA approval. These 15 patients did not show
device-related severe adverse events either. Another limitation was
the short follow up of the study. The original study design considered
most patients enrolled in this study to be terminal patients. A longer
follow up will be considered in our future studies.
Figure 5
Figure 6
Figure 6
MR image showing the gas bubbles near the interfaces between
the treatment table and the gel pad.
Table 1
Figure 7
Figure 7
MR image showing the calibration of the focal spot: (a) before and
(b) after the calibration on the coronal view, (c) axial view, and (d) sagittal
view.
Figure 8
Figure 9
Figure 9
The pain score after the MRgFUS treatment. One patient did not
return after one month for follow up, and another patient did not return after
two months for follow up.
Conclusion
In this work, the clinical workflow and practical QA procedures have been described for the palliative treatment of metastatic bone patients using the clinical MRgFUS system. Our data suggests that with careful execution of the QA procedures MRgFUS is a safe, effective and noninvasive treatment modality for metastatic bone palliation.
References
- Hill CR, ter Haar GR. Review article: high intensity focused ultrasound--potential for cancer treatment. Br J Radiol. 1995; 68(816): 1296-1303.
- Wu F, Wang ZB, Chen WZ, Zhu H, Bai J, Zou JZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004; 11(12): 1061-1069.
- Furusawa H, Namba K, Thomasen S, Akiyama F, Bendet A, Tanaka C, et al. Magnetic Resonance-Guided Focused Ultrasound Surgery of Breast Cancer: Reliability and Effectiveness. J Am Coll Surg. 2006; 203: 54-63.
- Furusawa H, Namba K, Nakahara H, Tanaka C, Yasuda Y, Hirabara E, et al. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS). Breast Cancer.2007; 14: 55-58.
- Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging--guided focused ultrasound. Radiology. 2008; 249: 355-363.
- Chen L, Ma CM, Richardson T, Freedman GM and Konski A. Treatment of Bone Metastasis Using MR Guided Focused Ultrasound. Medical physics. 2009; 36: 2486.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009; 34: 584-589.
- Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009; 16: 140-146.
- McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010; 66: 323-32.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010; 4: 294-302.
- Ritchie RW, Leslie T, Phillips R, Wu F, Illing R, ter Haar G, et al. Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up. BJU Int. 2010; 106: 1004-1009.
- Chen L, Mu Z, Hachem P, Ma C M, Pollack A. Enhancement of Drug Delivery in Prostate Tumor in vivo Using MR Guided Focused Ultrasound (MRgHIFU). Biomed Imaging Interv J. 2009; 341-344.
- Song W, Jung US, Suh YS, Jang HJ, Sung HH, Jeon HG, et al. High-intensity focused ultrasound as salvage therapy for patients with recurrent prostate cancer after radiotherapy. Korean J Urol. 2014; 55: 91-6.
- Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Ultrasonically Activated Chemotherapeutic Drug Delivery in a Rat Model. Cancer Res. 2002; 62: 7280–7283.
- Yuh EL, Shulman SG, Mehta SA, Xie J, Chen L, Frenkel V, et al. Delivery of systemic chemotherapeutic agent to tumors by using focused ultrasound: study in a murine model. Radiology. 2005; 234: 431-437.
- Khaibullina A, Jang BS, Sun H, Le N, Yu S, Frenkel V, et al. Pulsed high-intensity focused ultrasound enhances uptake of radiolabeled monoclonal antibody to human epidermoid tumor in nude mice. J Nucl Med. 2008; 49: 295-302.
- Chen L, Mu Z, Hachem P, Ma C-M, Pollack A. MR-guided focused ultrasound: enhancement of intratumoral uptake of [3H]-docetaxel in vivo. Phys Med Biol. 2010; 55: 7399-410.
- Chen X, Cvetkovic D, Ma C-M, Chen L. Quantitative Study of Focused Ultrasound Enhanced Doxorubicin Delivery to Prostate Tumor In Vivo with MRI guidance Med Phys. 2012; 39(5): 2780-2786.
- Rapoport NY, Nam KH, Gao Z, Kennedy A. Application of Ultrasound for Targeted Nanotherapy of Malignant. Tumors Acoustical physics. 2009; 55: 594-601.
- Mu Z, Ma C-M, Chen X, Pollack A, Chen L. MR Guided Pulsed High Intensity Focused Ultrasound Enhancement of Docetaxel Combined with Radiotherapy for Prostate Cancer Treatment. Phys Med Biol. 2012; 57: 535–545.
- Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer. Inst. 2014; 106(5).
- Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases--preliminary clinical experience. Ann Oncol. 2007; 18(1): 163-167.
- Peters RD, Hinks RS, Henkelman RM. Ex vivo tissue-type invariability in proton-resonance frequency shift MR thermometry. Magn Res Med. 1998; 40: 454–459.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984; 10(6): 787-800.