Case Report
Melanoma in Children
Sami H1*, Zayane A1, Naqos N2, Ahouissoussi C3, Bendouro H1, Rais H3, Belbaraka R2, Elomrani A1 and Khouchani M1
1Department of Radiation Oncology, Mohammed VI University Hospital, Marrakech, Morocco
2Department of Medical Oncology, Mohammed VI University Hospital, Marrakech, Morocco
3Department of Anatomopathology, Mohammed VI University Hospital, Marrakech, Morocco
*Corresponding author: Sami H, Department of Radiation Oncology, Mohammed VI University Hospital, Marrakech, Morocco
Published: 08 May, 2017
Cite this article as: Sami H, Zayane A, Naqos N,
Ahouissoussi C, Bendouro H, Rais H,
et al. Melanoma in Children. Clin Oncol.
2017; 2: 1281.
Abstract
Melanoma is a highly malignant skin cancer that begins in melanocytes. Many preexisting
conditions increase the risk of development of melanoma during childhood. These include giant
congenital melanocytic nevi, the familial dysplastic nevus syndrome, and xeroderma pigmentosum.
The signs and symptoms associated with melanoma in children are similar to those in adults, as well
as the histopathologic features, biologic behavior, and treatment of this tumor. Treatment is mainly
based on surgery at the localized stages. For metastatic melanoma, the therapeutic options are
chemotherapy, immunotherapy and targeted therapy. We report a new case of a 6-years-old child
with Xeroderma pigmentosum, who was operated several times for face and back tumors, who’s
morphological and immunohistochemical study confirmed the appearance of a melanoma. We also
report recent data from the literature on this subject by describing the features of melanoma in
children and the associated problems in order to facilitate an early diagnosis and thus an appropriate
therapeutic management.
Keywords: Melanoma; Child; Xeroderma Pigmentosum; Skin
Background
Melanoma is very rare in children. It represents 1 to 4% of the malignant tumors in the pediatric population [1]. There has been a steady increase in the incidence since the 1970s, particularly in the 15-19 age group [2]. It is often diagnosed late because of its atypical form in children [3,4].
Case Report
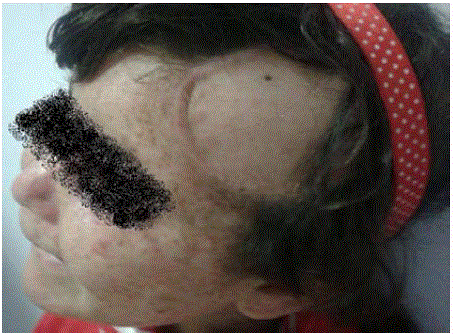
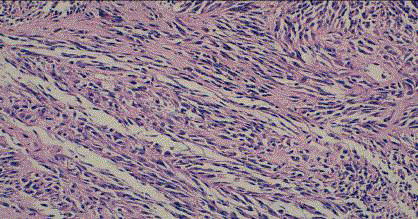
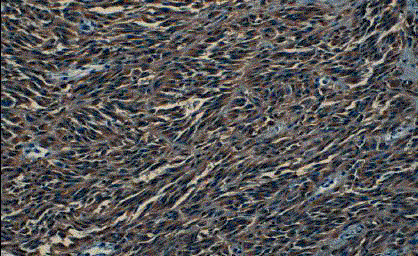
A 6 years old female child with xeroderma pigmentosum who was operated several times in the plastic surgery department (on the face and back). The last excision was done for of a lesion at the forehead that evolving for 8 months (Figure 1). The macroscopic examination revealed a fusiform cell melanoma with a thickness of 2cm according to Breslow and level IV of Clarck and Mihm (Figure 2 and 3). A surgical resumption was made 3 months later. At the histological examination, it was seat of discrete fibrous rearrangements without tumor residue. Then, the child was sent to the oncology department. The clinical examination did not record any lymphadenopathy. The chest, abdomen and pelvis Computed Tomography (CT) scan was normal. Surveillance was adopted in the absence of indication for adjuvant therapy.
Discussion
The current incidence of childhood melanoma is much less known for the child compared to
adult. However, adolescents aged from 15 to 19 years old were the most affected (73.2% of the
cases), followed by 10 to 14 year old (17.3% of cases), then 5 to 9 year old (5.7% of cases) and finally
1 to 4 years (3.8%) [5]. Wong JR “et al”. [6]. Studied the incidence of melanoma in the United
States between 1973 and 2009 using data collected by the Surveillance, Epidemiology and End
Results program and showed that 1,230 white-skinned children were diagnosed of melanoma and
increasing the incidence of pediatric melanoma by 2% per year. The incidence was significantly
higher in girls aged 15 to 19 than in boys and younger children. In boys, the incidence is higher
for the face and torso while in girls the incidence is higher for the lower limbs and hips [2].
Another study in the United States carried out between 2000 and 2010 counting 1185 children,
found that: the incidence of melanoma increases with age. Girls are more often affected than boys
except between 0 and 4 and between 10 and 14 years of age, where both sexes are affected equally.
The whites are mostly concerned (96.96%). The tumors are located mainly on the trunk between
15 and 19 years of age, on the trunk, head and neck between 10 and 14 years, at the extremities (hands feet) between 5 and 9 years, and on the head and neck for the younger ones [5]. Approximately 60% of melanomas have a
BRAF V600 mutation and 20% have an oncogenic NRAS mutation
[6], leading to the development of targeted therapies directed against
BRAF. However, these mutations alone cannot explain the genesis
of tumor, as evidenced by their presence in acquired, congenital or
spitzoid nævi [7,8]. The occurrence is possible on a congenital nevus,
especially if it is giant, or a dysplastic nevus, in a context of Xeroderma
pigmentosum (risk X 2000, average 13 years in boys and 20 years in
girls, especially head and neck) Clear photo type or exposure to UV
rays. Otherwise, immunosuppression syndromes, whether acquired
or not, increase the total number of nævi, a neoplastic history
(leukemia, retinoblastoma) or family history in 5% to 10% of infantile
cases [9]. All types of melanoma can be seen in children. They are
often of rapid growth and their diagnosis proves difficult, given their
often atypical appearance. The child's melanomas are often spitzoid,
nodular or unclassifiable [3,4,9]. The authors have developed ABCDE
criteria for children, to be used in addition to those already existing
and which should significantly increase sensitivity [10] (Table I).
In most studies, especially in prepubertal children, their tumor
thickness is significantly higher than that observed in adolescents and
adults [9-11]. This is particularly important because, apart from the
lymph node status, tumor thickness is the main prognostic factor for
melanoma [12]. Therefore, the child's melanoma is of bad prognostic:
at the 1-2-year stage at 10 years is 90%, vs. 60.1% at stage 3 and 30% at
5 years if advanced form, age >10 years. At equal thickness, pediatric
melanomas often have the same prognosis as those of adults [13-15].
The first stage in the treatment of melanoma, as in adults, is
complete and wide excision with margins that vary with depth
infiltration. For Stages I and II (absence of metastasis irrespective of
the depth of invasion), only a broad lesion of the lesion is consensual.
The indication of an adjuvant treatment in localized melanoma is
discussed. It can be considered for melanomas with high evolutive
risk (thickness according to Breslow >1.5mm, lymph node invasion
and histological ulceration of the primary tumor). Treatment of
regional lymph node metastases is radical lymph node dissection.
For locally advanced and metastatic stages, management involves
chemotherapy, biochemotherapy, immunotherapy or targeted
therapy [15-20]. The reference treatment among active drugs is
dacarbazine. Temozolomide also gives identical response rates
to dacarbazine, with the advantage of oral administration. The
chemotherapeutic agent compared to dacarbazine monotherapy
did not show superiority, but on the other hand a higher toxicity.
Interleukin 2 plus interferon plus Polychemotherapy trials have
been performed and appear to give a better response rate without
overall better survival, but this therapeutic regimen seems to be hard
to tolerate by infants [16,17]. For immunotherapy, ipilimumab, a
monoclonal antibody to the CTLA4 lymphocyte receptor has been
validated in metastatic melanomas, first in the second and then in
the first line [19]. The targeted therapy pathway was also opened
following the demonstration of the BRAF mutation and the approved
molecule was vemurafenib [18]. Systematic prophylactic excision of
giant congenital naevi is currently common in many centers due to
the risk of degeneration that often appears before 5 years. However,
the difficulty lies in the possibility of total lesion removal, in particular
at depth [21]. The 5-year survival rate varies from 50% to 76%. The
rate of recurrence after initial treatment is higher than in adults (52%
versus 40%), but these recurrences are often late (after 10 years)
[22,23].
Figure 1
Figure 2
Figure 3
Conclusion
The incidence of melanoma in children is steadily increasing.
Careful analysis of histologic features as well as the additional
information provided by immunohistochemistry should allow for a
correct diagnosis in most cases.
The epidemiology, the clinical and histological aspects are different from the adult, but the management of these melanomas
is similar.
References
- Hamre MR, Chuba P, Bakhshi S, Thomas R, Severson RK. Cutaneous melanoma in childhood and adolescence. Pediatr Hematol Oncol. 2002;19(5):309-17.
- Wong JR, Harris JK, Rodriguez-Galindo C, Johnson KJ. Incidence of childhood and adolescent melanoma in the United States: 1973-2009. Pediatrics. 2013;131(5):846-54.
- Han D, Zager JS, Han G. The unique clinical characteristics of melanoma diagnosed in children. Ann Surg Oncol. 2012;19:3888-95.
- Dinulos J. Childhood melanoma: what every pediatrician should know. Curr Opin Pediatr. 2009;21(4):472-4.
- Campbell LB, Kreicher KL, Gittleman HR, Strodtbeck K, Barnholtz-Sloan J, Bordeaux JS. Melanoma incidence in children and adolescents: decreasing trends in the United States. J Pediatr. 2015.
- Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol. 2011;164(4):776-84.
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33(1):19-20.
- Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol. 2007;127(1):179-82.
- Slade AD, Austin MT. Childhood melanoma: an increasingly important health problem in the USA. Current opinion in pediatrics. 2014;26:356-61.
- Cordoro KM, Gupta D, Frieden IJ, McCalmont T, Kashani-Sabet M. Pediatric melanoma: results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol. 2013; 68:913-25.
- Averbook BJ, Lee SJ, Delman KA, Gow KW, Zager JS, Sondak VK, et al. Pediatric melanoma: analysis of an international registry. Cancer. 2013;119(22):4012-9.
- Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735-41.
- Wechsler J. Melanoma during childhood. International Academy of Pathology (French Division).
- Jen M1, Murphy M, Grant-Kels JM. Childhood melanoma. Clin Dermatol. 2009;27(6):529-36.
- Livestro DP, Kaine EM, Michaelson JS, Mihm MC, Haluska FG, Muzikansky A, et al. Melanoma in the young: differences and similarities with adult melanoma: a case-matched controlled analysis. Cancer. 2007;110(3):614-24.
- Fraker DL. Management of in-transit melanoma of the extremity with isolated limb perfusion. Curr Treat Options Oncol. 2004;5(3):173-84.
- Buzaid A. Management of metastatic cutaneous melanoma. Oncology. 2004;18:1443-59.
- Chapman PB, Hauschild A, Robert A. Phase III randomized, open-label, multicenter trial (BRIM3) comparing BRAF inhibitor Vemurafenib with dacarbazine in patients with V600E BRAF mutated melanoma. ASCO. 2011.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-23.
- Réguerre Y, Avril MF, Fraitag S, Bodemer C. [Melanoma in children: diagnosis and treatment specificities]. Bull Cancer. 2012;99(9):881-8.
- Marghoob AA, Schoenbach SP, Kopf AW, Orlow SJ, Nossa R, Bart RS. Large congenital melanocytic nevi and the risk for the development of malignant melanoma. A prospective study. Arch Dermatol. 1996;132(2):170-5.
- http://anabible.webethan.org/spip.php?page=print-article&id_article=3666&lang=fr.
- Quatrano NA, Dinulos JG. Current principles of sunscreen use in children. Curr Opin Pediatr. 2013;25(1):122-9.