Case Presentation
Evolution of a System to Increase Precision in the Surgical Management of Colorectal Carcinoma
Charles L. Hitchcock1*, Thomas J. Magliery2, Cathy Mojzisik3, Morgan Johnson4, Mark W.
Arnold5 and Edward W. Martin6
1Department of Pathology, The Ohio State University, USA
2Department of Chemistry & Biochemistry, The Ohio State University, USA
3Clinical Development, Enlyton Ltd., USA
4The Ohio State University College of Medicine, USA
5Department of Surgery, The Ohio State University, USA
6Department of Surgery, The Ohio State University, USA
*Corresponding author: Charles L. Hitchcock, Department of Pathology, The Ohio State University, 1645 Neil Ave, Columbus, OH 43210, USA
Published: 05 May 2017
Cite this article as: Hitchcock CL, Magliery TJ, Mojzisik C,
Johnson M, Arnold MW, Martin EW.
Evolution of a System to Increase
Precision in the Surgical Management
of Colorectal Carcinoma. Clin Oncol.
2017; 2: 1280.
Abstract
Current surgical procedures for colorectal adenocarcinoma are plagued by a lack of precise information due to lack of sensitivity and specificity of preoperative imaging and surgical limitations in the Operating Room using traditional techniques (i.e., inspection and palpation). The staging of colorectal adenocarcinoma begins with preoperative imaging and ends with the pathologist. There are many potential sources of error between these two points that may result in suboptimal treatment. Using colorectal adenocarcinoma as a model, we developed a System incorporating currently available technologies to increase the precision of Preoperative and Intraoperative imaging as well as intraoperative tumor detection.
Introduction
The need for precision
Over 1,685,000 new cancers will be diagnosed in the U.S. in 2016, excluding keratinocyte
carcinoma. Of these, approximately 85% will be carcinomas with adenocarcinomas making up the
majority. Adenocarcinomas of the colon and rectum constitute 134,490 of these cases. However, the
prevalence of adenocarcinomas is four times the incidence rate, which equates to 621,430 patients
living with colorectal carcinoma in 2016 [1].
The National Comprehensive Cancer Network (NCCN) developed practice guidelines and
clinical resources to help physicians treat, diagnose, prevent, reduce risk, provide supportive care,
and image a large number of different cancers, including colorectal adenocarcinoma [2]. The TNM
staging criteria forms the platform for the guideline for colorectal carcinomas, and its accuracy is
critical to treatment selection and planning. The staging of these tumors begins with preoperative
imaging and ends with the pathologist, but there are many potential sources of error between these
two points that can impact patient treatment and outcome. In the case of colorectal carcinomas,
despite these evidence based guidelines, more than 40% of patients who underwent a “curative
resection” of a primary tumor have recurrent disease, and patients with the same stage of colorectal
adenocarcinoma can differ in their clinical course. These statistics occur due to a lack of precision.
The National Institutes of Health (NIH) defines the term “Precision Medicine” as “an emerging
approach for disease treatment and prevention that takes into account individual variability in
genes, environment, and lifestyle for each person” [3]. In the case of colorectal adenocarcinoma, the
complete removal of all tumor-bearing tissue requires precision in the localization and detection of
intraabdominal metastatic disease before and during surgery. There are several factors that impact
this precision.
The NCCN guidelines recommend using Computerized Tomography (CT) scans with contrast
for preoperative imaging. This assessment of extent of disease is needed for surgical planning for
resection of primary and recurrent disease. This includes resectability of the primary tumor and
assessment of the presence of metastatic disease that alters the surgical approach or mandates nonsurgical
therapies. Despite providing anatomic information, the specificity and sensitivity of CT imaging to detect lymph node metastases is limited by its inability to: 1) identify lymph nodes smaller than 5 mm that often contain
metastatic disease, 2) distinguish non-enlarged lymph nodes under a
centimeter containing tumor from normal physiologic non-enlarged
lymph nodes, and 3) distinguish enlarged lymph nodes containing
tumor from lymph nodes that are enlarged due to reactive/
inflammatory changes. [4] The end result is a wide range of reported
specificity from only 42% to 70% [5-9].
Patients and their families often ask “Did you get it all?” Current
surgical procedures are based on surgical anatomy and traditional
planes of resection that are easily violated by cancer cells. Variation
in surgeon experience influences the type of tumor resection and
surgical precision. Traditional surgical techniques (i.e. visual
inspection and palpation) do not necessarily provide surgeons with
accurate information regarding location and extent of disease needed
to obtain a “curative” resection. As one of us has previously noted,
“surgeons had real-time information regarding the precise location
of all disease and had a real-time assessment of surgical resection
margins, they may be able to intervene immediately and accomplish
a complete resection without subjecting the patient to subsequent
surgical procedures [10].
Advances in precision medicine are underway. This paper
examines how a diverse group of physicians, basic scientists and
engineers brought together currently available resources and new
developments into a multimodal System that provides the surgeon
with the approach and tools needed to increase the precision of
tumor imaging and detection before and during surgery for patient’s
solid tumors. Although the focus is colorectal adenocarcinoma, the
proposed System applies to the majority of adenocarcinomas that
arise in other organs.
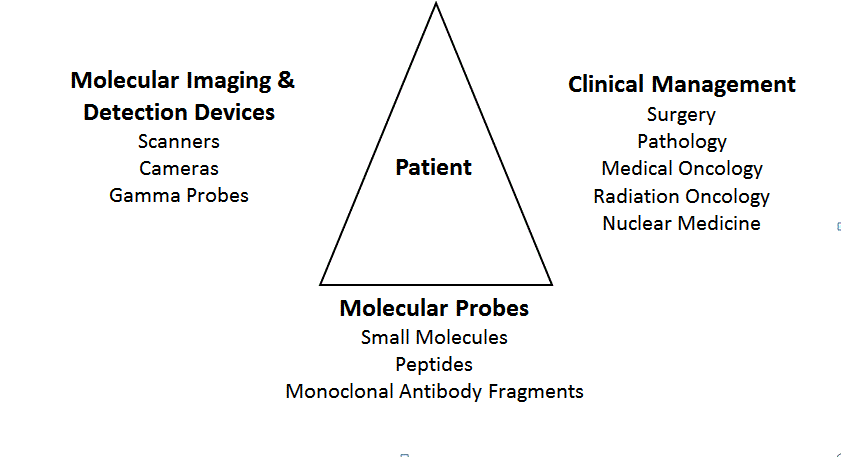
Figure 1
Figure 1
The System. The System begins with the patient and the solid
tumor. The tumor’s pathologic features are used to select the appropriate
tumor specific or associated molecular probe and radionuclide or nonradioactive
label. The labeled-molecular probe dictates the devices that
can be used for preoperative and intraoperative imaging and intraoperative
detection. The results will aid in treatment decision making before and/or
after tissue examination by Pathology.
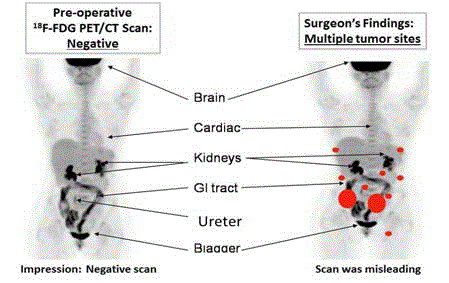
Figure 2
Figure 2
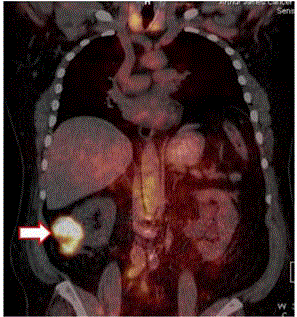
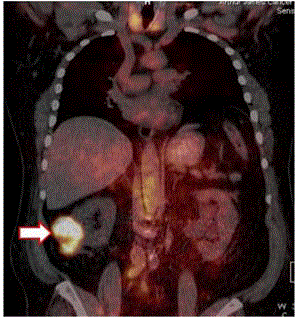
18F-FDG PET/CT of Patient with Recurrent Colon Cancer.
The left image is the pre-operative PET/CT scan was interpreted as negative
for cancer. Nonspecific uptake of the 18F-FDG was present in the brain, GI
tract, kidneys, ureter and bladder. The right image correlates the surgical
findings of cancer (orange dots) with the same 18F-FDG-PET/CT scan.
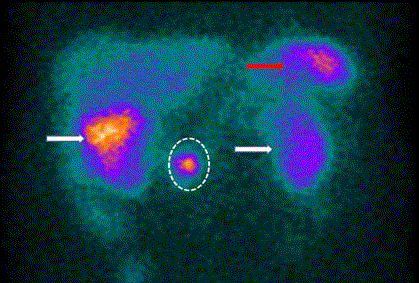
Figure 3
Figure 3
SPECT/CT Scan of 111In-pentetreotide-positive gastrinoma.
Large field-of-view gamma camera (SPECT) scan of 111In-pentetreotide
bound to somatostatin receptors on a gastrinoma cells (dotted circle). There
is non-specific uptake in the spleen (red arrow) and accumulation in the
gallbladder (right white arrow), and in the kidneys (left kidney- white arrow,
right kidney behind the gallbladder). Note the poor spatial resolution.
Methods
A System to increase precision management of colorectal cancer patients
System components: The components of the multimodal System
are seen in Figure 1. With the patient at its center, the System integrate
s physicians from Nuclear Medicine, Radiology, Surgery, Oncology,
Radiation Oncology and Pathology with the tools needed for a more
precise diagnosis and treatment of the patient’s cancer. Molecular
probes, specific for the patient’s tumor, are the foundation of the
System. Based on the results of the initial biopsy and/or laboratory
studies, the Pathologist recommends the appropriate molecular
probe to be used. Labeling of the molecular probe is dictated by the
type of molecular imaging and intraoperative detection devices. The
results of molecular imaging determine optimal treatment, such as
surgery or undergoing chemotherapy and/or radiation therapy.
Precise imaging provides the surgeon with a “mine field map” for
intraoperative detection using a hand-held gamma detection probe
to find and excise/resect the tumor containing tissue. Intraoperative
imaging provides real-time verification of complete resection. From
a systems standpoint, the complete resection of all tumor is globally
cost effective.
Molecular probes: In contrast to the anatomic information
provided by CT and MRI imaging, molecular imaging is a diagnostic
modality that provides functional information about molecular
makeup of tissue. Molecular imaging uses a variety of radiolabeled
molecular probes for Positron Emission Tomography (PET) and
Single-Photon Emission Computed Tomography (SPECT) alone or
in combination with CT or Magnetic Resonance Imaging (MRI).
Constantly evolving, molecular imaging provides the necessary
versatility needed for the System’s multimodality approach to increase
the precision of cancer surgery [11]. There are several categories of
tumor-related molecular probes available for molecular imaging [12].
They include small molecules that bind intracellular targets, small
peptides that bind to membrane receptors, Monoclonal Antibodies (MAbs) and bioengineered MAb fragments that bind to tumorrelated
antigens. Ongoing studies are directed at the production of
molecular probes that rapidly bind to the specific target in the tumor,
lack uptake by non-target tissue, and rapidly clear from the blood and
normal tissue. The end result of this optimization is a reduction in
unwanted background that will yield the maximum signal-to-noise
for the probe [13].
Categorized as a small molecule molecular probe, [18F]-2-fluoro-
2-deoxyglucose (18F-FDG) is widely used for preoperative PET or
PET/CT imaging of patients with cancer, monitoring patients for
recurrent disease, and more recently for assessing response to therapy
[14]. However, 18F-FDG is not cancer specific. As a glucose analog,
FDG is taken up into cells with a high metabolic rate. This includes
cells within malignant and some benign tumors, normal organs (e.g.,
brown fat, myocardium and other muscle, brain, gastrointestinal
(GI) tract, thyroid, liver and spleen), inflammatory responses (e.g.,
infections, granulomas, and immune hyperplasia), and wound
healing. In addition, FDG accumulates in the kidneys and bladder due
to its excretion in the urine [15]. Uptake of FDG as described results
in false positive findings. In addition, tumors with a low metabolic
rate do not take up FDG. False negative PET and PET/CT scans
often occur with well differentiated adenocarcinomas of the lung
such as invasive bronchioloalveolar carcinomas, carcinoid tumors
in the lung, renal cell carcinomas, hepatocellular carcinomas in the
liver, mucinous tumors of the gastrointestinal tract, and low grade
non-Hodgkin lymphomas. The false positive and false negative rates
limit the precision of 18F-FDG PET and 18F-FDG PET/CT for preor
perioperative staging of tumors [16,17]. The reported sensitivity
of 18F-FDG PET/CT for the detection of lymph node metastases is
reported as low as 43% for colorectal carcinomas, which is below the
needed diagnostic precision for the System (Figure 2) [18].
Small peptides of ≤15 amino acid are used for both SPECT
and PET molecular imaging, alone or in combination with CT.
These molecular probes act as ligands for various membrane
receptors, the most common of which are somatostatin receptors
on Neuroendocrine Tumors (NETs). They have excellent specificity,
stability, and low immunogenicity, but are prone to proteolysis
[12,19,20]. Radionuclide labeling generally requires an intermediate
chelator attached to the peptide. As most gastrinomas and other
foregut neuroendocrine tumors overexpress somatostatin receptors,
somatostatin receptor imaging using SPECT/CT is the method-ofchoice
for pre- and/or perioperative staging of gastrinomas (Figure 3)
[21, 22]. However, the advent of PET/CT probes may replace them in
the future, especially for midgut and hind gut NETs [23].
Monoclonal Antibodies (MAb), directed against tumor-related
antigens, are being developed as molecular probes for molecular
imaging, intraoperative detection, and/or therapy. The efficacy
of a given MAb is limited by the type of tumor(s) and the level of
expression of the target antigen. Ideally, MAb molecular probes
exhibit the properties listed in Table 1. Several different MAbs have
been approved by the FDA for molecular imaging [12], the majority
of which have been labeled for SPECT imaging. However, numerous
other monoclonal antibodies and their bioengineered counterparts
are working their way through the clinical trial steps needed for Food
and Drug Administration (FDA) approval for molecular imaging
both SPECT 123I and PET 124I modalities.
The development of MAbs to meet these desired properties
resulted in multiple generations of monoclonal antibodies and their
biochemical and genetic engineered protein fragments of antibody
molecules (Figure 4). The clinical utility of first generation intact
murine IgG molecules was limited by their: large size, accumulation
in non-target tissue, long serum half-lives, and immunogenicity
that induced the formation of Human Anti-Mouse Antibodies
(HAMA). Slow clearance and uptake in the target tissue necessitated
radiolabeling with radionuclides with longer half-life isotope [111In
(2.8 days), 89Zr (3.3 days), 124I (4.2 days), or 125I (60 days)] [24].
Enzymatic digestion of the intact IgG molecules gave rise to smaller
F(ab)2 and Fab fragments with better pharmacokinetics; however,
these were still immunogenic. Genetic engineering directed at
minimizing the immunogenicity resulted in chimeric IgG molecules
(Figure 4) that contain amino acids of the murine variable regions
attached to the human constant regions. Fully humanized MAbs (not
shown) containing only 5% murine-derived amino acids from the
antigen binding site [25].
Development of MAb fragments for the desired properties of a
given application resulted in small single-chain variable fragments
(scFv) and their diabodies. The scFv is monomeric with a 12–15
amino acid linker (Figure 4 - red line) between the VH and the VL
domains. Linker composition and length can have a significant
impact on antigen binding and stability. Diabodies contain two
non-covalently associated scFv-like fragments that interact with and
bind to their corresponding antigen in a divalent manner. Tribody
and tetrabody molecules of these scFv fragments are also possible.
The scFv fragments of bispecific diabodies (not shown) have different
antigen binding specificities. When compared to intact IgG, F(ab)2
and Fab fragments, scFvs and diabodies have faster clearance with
excellent tumor penetration and higher tumor-to-blood ratios. The
low background and high signal-to-noise ratio increases the precision
of molecular imaging to identify malignant tissue [26,27].
Tuning antibody fragments to the exact molecular imaging
application remains a significant frontier for engineering and
development. The fragment size can be adjusted by genetic
engineering, linker manipulation, and chemical modification with an
inert Polymer of Ethylene Glycol (PEG), but often these modifications
result in poor stability, poor or ablated binding, and aggregation.
However, adjustments in fragments size translate into adjustments
in clearance time suitable for different imaging time lines, modalities
and sensitivities.
Molecular imaging and intraoperative detection devices
Detection of tumor-related molecular probes depends on the
use of a wide range of radionuclides and non-radioactive labels. The
half-life of the radionuclide must be matched to the half-life of the
molecular probe to optimize imaging and timing of surgery. As an
example, if a particular molecular probe is slow to clear from the
blood and normal tissue, then the imaging is delayed for several days
or weeks, and the radioisotope with a shorter half-life would not be
detected. PET imaging requires positron emitting radionuclides,
whereas SPECT imaging directly detects photons from gamma
emitters.
High energy (511 KeV) radionuclides such as 18F, 124I or 68Ga emit
positrons that annihilate electrons, giving rise to two photons that
travel in opposite directions and are detected by the PET scanner. PET
instruments contain multiple gamma cameras arranged in a circular
fashion. PET is now typically combined with CT for anatomical
information. Lower energy radionuclides such as 123I, 99mTc, and 111In
emit γ-radiation which is detected using planar or tomographical
γ-cameras (SPECT). The ability to perform whole body scans and
obtain multiple images over time is a major advantage of these types of
molecular imaging. The limitless depth of penetration associated with
the imaging use of radionuclide-labeled molecular probes induces a
loss of spatial resolution due to the inverse square law of intensity as
a function of distance. Combining CT or MRI with PET or SPECT
along with the ongoing development of new generations of tumor–
specific MAbs will only increase the precision of molecular imaging
by providing both anatomic and more precise functional localization
of primary and metastatic malignancies. For example, tumor–specific
MAbs labeled with high energy molecular probes have been shown to
provide high specificity and sensitivity in detecting tumors in patients
with clear cell renal cell carcinoma [28] (Figure 5).
Hand-Held Gamma Detection Probes (HGDPs), and to a
lesser extent laparoscopic gamma detecting probes, are used for
intraoperative detection of radiation that is unbound or bound to a
molecular probe [24,29,30]. Widely available, these probes are either
like a gamma camera, or they are solid state detectors containing a
semiconductor crystal. Our studies have primarily employed a HGDP
containing a cadmium telluride (CdTe) crystal linked to a control
unit that provides both numerical information and an auditory
signal when the radioactivity is higher than three standard deviations
above the background radiation [31]. Their precision for routine
use in radioguided surgery is operator dependent. The surgeon may
not go outside of the planned surgical field, may not be aware of the
instrument’s restricted field of view, or understand the sensitivity
and specificity increases as the probe moves closer to the source
of radiation [24]. The precision of the surgeon using the HGDP, is
enhanced by utilizing an intraoperative portable gamma camera
that provides real-time intraoperative localization of low-energy
radionuclide labeled molecular probes. The use of these nuclear
medicine instruments allows the surgeon in real-time to determine
the success of the operation and whether or not he or she “got it all.”
Commercially available portable gamma cameras collect the
low energy emission to produce a planar image that can be used in
surgery to provide real-time images. Small gamma cameras are handheld
and are easily used for intraoperative imaging. However, these
instruments take 10-60 seconds to generate an image which may be
less than optimal due to an unsteady hand. Larger, portable, gamma
cameras require stabilization and can have either a small field of view
(5cm2 x 5cm2) or large field of view ( >5 cm2 x5cm2) such as seen in
Figure 3 [32]. We and others have used intraoperative gamma cameras
for intraoperative imaging of sentinel lymph nodes, parathyroid
adenomas, and a variety of tumors including: gastrinomas, head-andneck
squamous cell carcinomas, breast cancer, and melanoma [28,32-
34]. Gamma cameras have a larger field of view than the HGDP and
thus provide the surgeon with a unique visual assessment of the
extent of disease and its complete resection.
Table 1
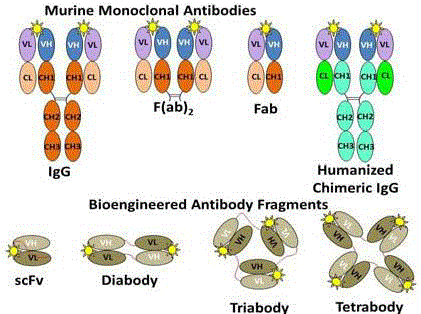
Figure 4
Figure 4
Types of Antibody-Derived Molecules for Molecular Imaging
and Intraoperative Detection.
Yellow star represents the same antigenic epitope attached to the antigen
binding site of each of the MAb molecules and their fragments. The murine
variable regions are fused to human constant regions to give rise to the
humanized and chimeric IgG molecules. The scFv and its corresponding
diabody, triabody, and tetrabody variants can contain only a few murinederived
amino acids, which are essential for antigen binding. The peptide
linker between the domains provides many of the desired physical properties
of these molecular probes for imaging.
Figure 5
Figure 5
Molecular Imaging of Clear Cell Renal Cell Carcinoma.
124I MAb cG250 PET/CT with clear cell renal cell carcinoma in the lower pole
of the right kidney (arrow). Focal molecular probe also labels the thyroid
glands.
Table 2
Table 3
A System Engineered to Increase Surgical Precision for Colorectal Carcinoma
Based on initial conventional imaging studies, up to 80% of
patients with colorectal adenocarcinoma lack clinical stage IV disease
and undergo curative surgery with or without adjuvant therapy (Table
2). However, more than 40% of these patients will have recurrent
disease, which primarily occurs in the lymph nodes, liver and/or
lungs. The best survival potential for patients undergoing curative
surgery for colorectal adenocarcinoma is the complete removal of all
tissue containing tumor.
The proposed “System” brings together the surgeon, radiologist,
nuclear medicine physician, and pathologist in order to increase
the precision of “getting it all.” Using molecular imaging, they
identify where the malignant tumor sites are and intraoperatively
refine the “map” to ensure that the surgeon does a more complete
resection. Increasing the precision of intraoperative detection of
tumor will increase the pathologist’s ability to “physiologically,” as
well as anatomically, stage the tumor. In the last 35 years, our group
generated several lines of evidence supporting this clinical claim,
especially for colorectal adenocarcinomas.
The “System” begins with the selection of the most appropriate
tumor-related antigen. For colorectal carcinoma we selected Tumor
Associated Glycoprotein-72 (TAG-72). TAG-72 is an oncofetal
antigen expressed by the majority of human adenocarcinomas
(Table 3). TAG-72 is a large mucin-like molecule consisting of 80%
carbohydrate moieties [27]. Immunohistochemical staining for TAG-
72 (Figure 6) demonstrates these molecules in cytoplasmic vacuoles
of the tumor cells that release the molecule into the lumen of tumor
acini and extracellular matrix where it accumulates. The extracellular
accumulation of TAG-72 facilitates its targeting by radiolabeled
antibodies and subsequent localization by molecular imaging and
hand-held probes. These features result in the ideal target molecule
for molecular imaging and intraoperative detection.
The TAG-72 molecule is a complex array of different antigenic
epitopes, to which multiple MAbs have been developed [38]. Of
these, we selected B72.3 murine MAb and its subsequent generations.
The evolution of antibodies to TAG-72 followed the prescribed path
previously noted for MAbs as molecular probes (Table 4). The initial
four generations of antibodies to TAG-72 were generated in the same
laboratory at the National Cancer Institute (NCI) [39-41], and were
used by us to increase the precision of radioimmunoguided surgery
(RIGS) in an attempt to detect all Tag-72 bearing tissue in real-time
and to remove it from patients with either primary or recurrent
colorectal adenocarcinoma [29].
Clinical studies using the first three generations of the murine
anti-TAG-72 MAbs were complicated by several factors. The
immunogenicity of murine IgG molecules resulted in development
of HAMA, whose only clinical significance was interference with
several clinical laboratory tests [42]. The fact that these were whole
IgG molecules with a long half-life required labelling with 125I with
half-life of 60 days resulted in a delay of surgery up to four weeks
and a tumor-background (signal-noise) ratio of 2:1. The smaller size
of the 3rd generation MAb doubled the tumor-background ratio and
halved its clearance time to allow for an improved time to surgery,
and did not induce significant HAMA [43,44]. One of these first
three generations of 125I-labelled MAbs to TAG-72 to study over
1,000 patients with either primary or recurrent adenocarcinomas,
with a focus on colorectal carcinomas. (Reviewed in 29, 31) Figure
7 demonstrates the increased precision by which the surgeon can
detect remove TAG-72 containing metastatic disease using a HGDP as compared to that obtained by traditional visual inspection and
palpation [45]. These findings, found in numerous other studies
[46-50], had significant impact in altering clinical decision making
in up to 50% of cases. These decisions included abandoning surgery
due to extensive disease (e.g., carcinomatosis), increasing the area of
resection, and up-staging leading to adjuvant chemotherapy [45-54].
The increased precision of intraoperative detection and removal
of occult metastatic disease provides a significant survival advantage
to patients with primary colorectal adenocarcinoma (Figure 8).
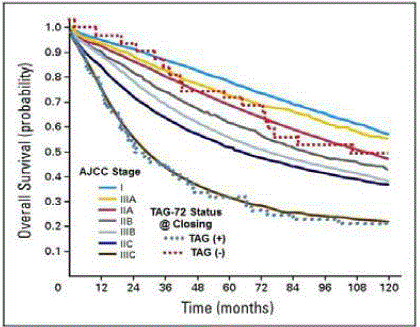
A longitudinal follow-up of 97 patients with primary colorectal
adenocarcinoma demonstrated that patient survival at 5, 10, and 15
years [31,55,56] was significantly improved when all of the TAG-72
positive tissue was surgically removed. The TAG-72 status at the end
of surgery is a bimodal, real-time, intraoperative assessment of the
patient’s survival potential at the time of closing that is independent
of the TNM stage. The survival of those patients in the TAG (+)
category mimics that of patients with Stage IIIC disease. In contrast,
for those patients in whom all TAG-72 containing tissue was removed,
classified as TAG (-), regardless of the TNM stage, the survival was
consistent with disease confined to the bowel wall with or without
minimal nodal involvement.
TAG-72 positive tissue that lacks evidence of tumor on routine
H&E staining is considered to be a false-positive finding [50,58].
However, several lines of evidence indicate that this is a misconception.
Clinically, the data in Figure 8 indicate that all TAG-72 positive
tissue, regardless of H&E staining status, has clinical significance if
left behind. Secondly, the non-regional periportal lymph nodes often
contain TAG-72 activity with the HGDP. Subsequent recurrent
disease was found in these nodes if they had not been previously
resected [59]. Just as important, routine pathologic examination
of these “false positive” lymph nodes lacks precision. Additional
sections submitted for H&E staining and/or immunohistochemical
staining did demonstrate metastatic disease, though the detection
sensitivity of the light microscope appears to have its limits as well
[60-62]. More sensitive molecular studies detected metastatic cells
where the microscope could not [63,64].
Many of these previous studies were complicated by the use of the first three generations of murine MAbs to TAG-72. They were
potentially immunogenic and their large molecular size resulted in
poor pharmacokinetics and the need for 125I labelling with its less
than optimal long half-life that delayed surgery up to four weeks after
injection [27,63]. Despite these obvious disadvantages, the precision
in the surgical management of colorectal adenocarcinoma, as well
as other tumors, can be further increased by using the previously
mentioned (above) multimodal approach, where the tumor-related
antigen TAG-72 is targeted using 5th generation scFv or other
fragment MAbs, labelled with radionuclides 123I or 124I with their
short half-lives. The excellent pharmacokinetics of these molecules
provide little background to impair preoperative and/or perioperative
molecular imaging while facilitating next-day-surgery using a HGDP
and intraoperative and post-operative molecular imaging.
This can be accomplished by targeting TAG-72 using humanized
single chain Fv fragments (scFv) and its bi- tri- and tetravalent forms
(Figure 4). These smaller molecules retain the specificity and affinity
of the previous generation murine CC49 (unpublished data). Their
small size optimizes their pharmacokinetics, yielding molecular
imaging with a much higher signal-to-noise (i.e., tumor-tobackground)
ratio (unpublished data) as well as providing for same
day surgery and intraoperative detection. Studies with xenografts
of human adenocarcinoma cells clearly demonstrate 18F-FDG and
the humanized 4th generation MAb to TAG-72 (3E8), yet lack the
precision obtained using humanized 3E8 fragment, a 5th generation
MAb to TAG-72 (Figures 9 and 10).
The clinical significance of this proposed approach has been
addressed in recent Proof-Of-Concept (POC) studies that combined
pre- and perioperative molecular imaging with intraoperative
imaging and the use of a HGDP to ensure that the surgeon “got it
all”. Gastrinomas are often characterized by over expression of
somatostatin receptors on their membrane which bind the peptide
ligand 111In-labeled octreotide as a molecular probe for imaging.
A POC study clearly demonstrated that the probe can be used for
preoperative SPECT/CT followed by planar imaging with a portable
Large Field-Of-View Gamma Camera (LFOVGC) before incision,
and at the completion of surgery, intraoperatively. The precision of
the surgery was furthered by the intraoperative use of a HGDP for
locating primary and metastatic tumors [28,34]. A second POC study
used the same approach for the molecular imaging 99mTc-Sestamibi
(MIBI) binding to parathyroid adenomas in 20 patients [33]. Although
a benign disease, primary hyperparathyroidism requires the resection
of the related parathyroid adenomas to prevent development of
debilitating sequelae. Resection of the involved gland is often
complicated by its variable location in the neck and mediastinum. The
portable LFOVGC was again used to ensure complete resection prior
to closure. The resulting increase in precision significantly decreased
time in the operating room by reducing the need to confirm complete
resection by delaying Parathyroid Hormone (PTH) studies until the
patient was in recovery.
Figure 6
Figure 6
Adenocarcinoma of the Colon - Immunohistochemical
Staining of the TAG-72 Antigen.
Anti-TAG-72 scFv with ABC immunohistochemical staining of a mucinous
adenocarcinoma. Extensive accumulation of TAG-72 in the extracellular
matrix is seen as dark brown staining (blue arrow). Intracellular TAG-72
containing vesicles exhibit a lesser staining intensity (red arrows). (20x
Virtual Slide).
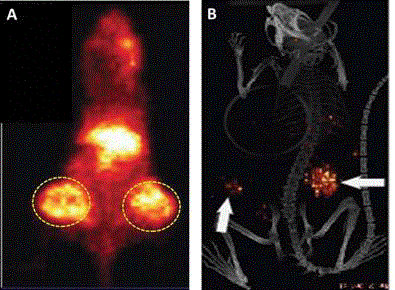
Figure 7
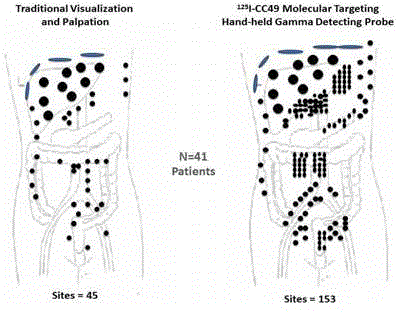
Figure 7
Intraoperative Tumor Location of Metastatic Disease.
Traditional Visualization and Palpation vs. Hand-Held Gamma Detection
Probe (HGDP) of Occult Tumor Binding 125I-CC49 in 41 Cases of Primary
Colorectal Adenocarcinoma [45]. The left drawing represents the 45
individual sites (black dots) of occult metastatic disease detected at traditional
visual inspection exploration of the abdomen and pelvis. The right drawing
demonstrates the 153 sites (black dots) of occult metastases found in the
same 41 patients with primary colorectal adenocarcinoma using a HGDP.
Figure 8
Figure 8
Ten Year Patient Survival: Traditional Surgery vs HGDP
Intraoperative Detection. Based on current AJCC TNM staging criteria,
the solid lines represent the 10-year survival for 128,853 primary colon
carcinoma patients in the SEER Database. (57) Using the presence [TAG
(+ - blue dotted line)] or absence [(TAG (-) – red dotted line] of radioactivity
at the time of closing (TAG-72 Status at Closing) the dotted lines represent
survival data from 97 patients that were given 125I-CC49 and subsequently
underwent HGDP directed intraoperative detection with possible resection of
radioactive tissue [56].
Figure 9
Figure 9
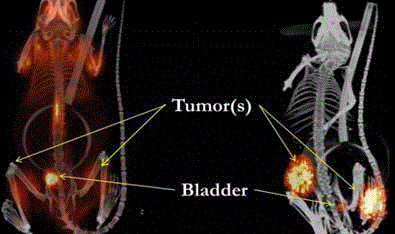
18F-FDG vs. 124I 3E8 fragment PET Scan Images of Human
Colon Cancer Xenografts in Mice.
The 18F-FDG-PET/CT (left image) was obtained 2 hours after injection.
The 124I 3E8 fragment PET/CT (right image) was obtained 24 hours after
injection. The tumor xenografts are clearly demonstrated in the 124I 3E8
fragment injected mouse image, whereas the 18F-FDG-PET image only
demonstrates physiologic excreted urinary activity in the bladder, nonspecific
activity in spine, and a diffuse background of nonspecific/physiologic activity
predominantly in muscle.
Figure 10
Figure 10
124I-IgG 3E8 PET Scan vs. 124I-diabody 3E8 PET/CT Scan of
Human Colon Cancer Xenografts in Mice.
The 124I-IgG 3E8 PET scan image A demonstrates a high background signal,
i.e., noise, with accumulation in the liver and head as compared to the
significantly lower background seen in the 124I-3E8 fragment PET/CT scan
image at 24 hours.
Figure 11
Conclusion
The current guidelines for colorectal cancer surgery do not take
into account the limited precision of Preoperative CT scans and
intraoperative visual inspection and palpation to accurately detect
nodal metastases outside of the traditional planes of dissection.
This lack of accurate information, has a significant impact on long
term survival. Despite its use as a molecular imaging agent, 18F-FDG
lacks accuracy in the identification of metastatic lymph nodes. The
identification and excision of malignant lymph nodes requires a
multimodal System. As proposed here, this System brings together
the necessary resources and the expertise of various clinical specialties
needed to present the surgeon with real-time intraoperative information needed to locate, identify and resect all malignant tissue
expressing the radiolabeled molecular probe. Two small proof-ofconcept
studies used this approach with great success; however, these
studies require expansion. The model system for these expanded
studies should be one where the number of potential patients is large
and the clinical impact can be determined with statistical confidence.
We propose that such a study be undertaken with primary colorectal
adenocarcinomas that examines the role of the proposed System on
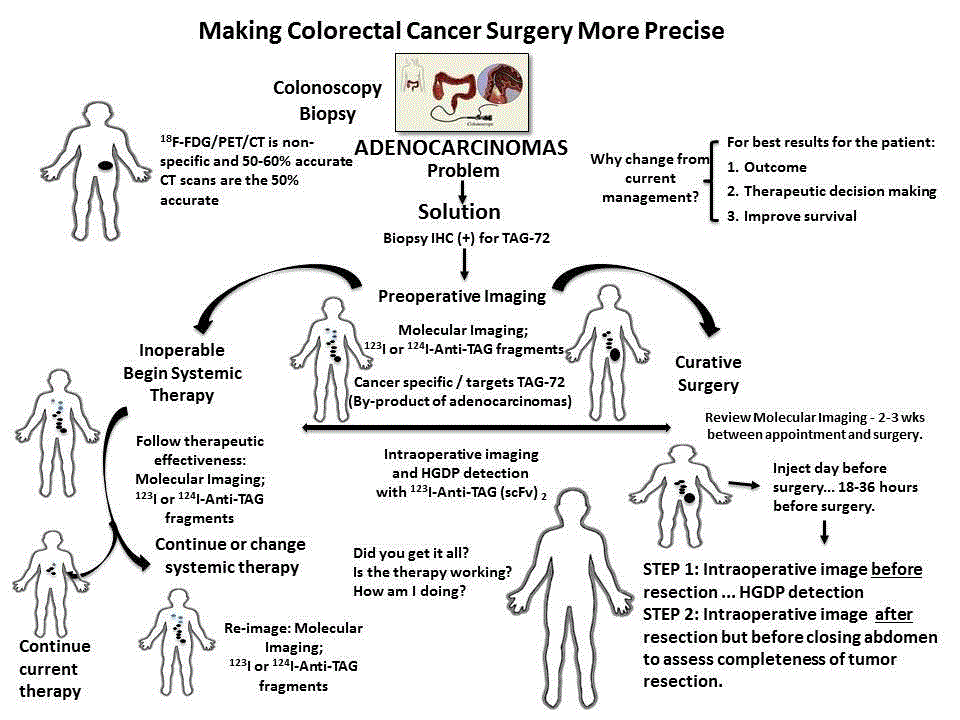
making colorectal cancer surgery more precise (Figure 11).
The initial workup for a patient presenting with colorectal
cancer is laboratory studies, including CEA serum levels, and
colonoscopy with biopsy. If an invasive adenocarcinoma is noted,
the pathologist will perform IHC staining to determine the presence
or absence of TAG-72 expression. The fact that TAG-72 is expressed
in 85% of colorectal adenocarcinomas makes anti-TAG-72 the ideal
foundational molecular probe for the System in these patients. If the
initial biopsy is shown to express TAG-72, the patient is injected
with 124I- or 123I-anti-TAG-72 antibody fragment and imaged using
PET/CT or PET/MRI, or SPECT/CT, respectively. The results of this
molecular imaging determine if the patient can undergo surgery for
cure or undergo chemotherapy and/or radiation therapy.
If clinically resectable, the day before surgery the patient is
given 123I-anti-TAG-72 fragment-cocktail to facilitate localization of
TAG-72 antigen-expressing malignant tissue. Intraoperative use of a
HGDP in conjunction with a portable LFOVGC allows the surgeon
to precisely identify all TAG-72 positive tissue, including surgical
margins for excision, and to ensure that it is excised. Prior to closing,
a planar image will tell the surgeon the patient’s TAG-72 status at
closing. This real-time intraoperative information about each tissue
specimen will be available to the pathologist to aid in clearly identifying
where to sample the resected specimens for subsequent processing
and microscopic examination. In addition, this information will
be available for more precise post-operative treatment planning
before the patient leaves the recovery room. If molecular imaging
demonstrates inoperable disease, the patient is referred to a medical
oncologist for treatment planning that may include chemotherapy
and/or radiation therapy. Here again the molecular imaging using
either 124I or 123I labeled anti-TAG-72 fragments will be used to
follow therapeutic effectiveness. This targeted approach will result in
increased identification and treatment of cancer and improve long
term survival for patients with a variety of types of cancer.
Table 4
References
- American Cancer Society, Cancer Facts & Figures 2016, Atlanta, American Cancer Society. 2016.
- www.nccn.org
- www.nih.gov/precision-medicine-initiative-cohort-program
- Herrera-Ornelas L, Justiniano J, Castillo N, Petrelli NJ, Stulc JP, Mittelman A. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987; 122(11): 253-6.
- Thoeni RF. Colorectal cancer. Radiologic staging. Radiol Clin North Am. 1997;35:457-85.
- Jeune F, Brouquet A, Caramella C, Gayet M, Abdalla S, Verin AL, et al. Cardiophrenic angle lymph node is an indicator of metastatic spread but not specifically peritoneal carcinomatosis in colorectal cancer patients: Results of a prospective validation study in 91 patients. Eur J Surg Oncol. 2016;42:861-868.
- Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A'Hern R, et al. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol. 2010;65:708-19.
- Wiegering A, Kunz M, Hussein M, Klein I, Wiegering V, Uthe FW, et al. Diagnostic value of preoperative CT scan to stratify colon cancer for neoadjuvant therapy. Int J Colorectal Dis. 2015; 30(8):1067-73.
- de Vries FE, da Costa DW, van der Mooren K, van Dorp TA, Vrouenraets BC. The value of pre-operative computed tomography scanning for the assessment of lymph node status in patients with colon cancer. Eur J Surg Oncol. 2014; 40(12):1777-81.
- Edward W. Martin, Jr. Forward, In K. Herrmann, O. E. Nieweg, and S.P. Povoski (Eds.), Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice (pages vii-ix). Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016.
- Weber J, Haberkorn U, Mier W. Cancer stratification by molecular imaging. Int J Mol Sci. 2015;16(3):4918-46.
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Phys Reviews. 2012; 92:897-965.
- Frangioni JV. The problem is background, not signal. Molecular Imaging. 2009;8(6):303–304.
- Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, et al. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1-192.
- Long NM, Smith CS. Causes and imaging features of false positives and false negatives on 18F-PET/CT in oncologic imaging. Insights Imaging. 2011; 2: 679–698.
- Lu YY, Chen JH, Ding HJ, Chien CR, Lin WY, Kao CH. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18F-FDG PET or PET/CT. Nucl Med Commun. 2012; 33:1127-1133.
- Park K, Jang G, Baek S, Song H. Usefulness of combined PET/CT to assess regional lymph node involvement in gastric cancer. Tumori. 2014;100(2):201-6.
- Gade M, Kubik M, Fisker RV, Thorlacius-Ussing O, Petersen LJ. Diagnostic value of (18)F-FDG PET/CT as first choice in the detection of recurrent colorectal cancer due to rising CEA. Cancer Imaging. 2015; 15(1):11.
- Andreas Kjaer, Ulrich Knigge. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand J Gastroenterol. 2015; 50(6):740–747.
- Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10(14):225977.
- Béhé M, Gotthardt M, Behr TM. Imaging of gastrinomas by nuclear medicine methods. Wien Klin Wochenschr. 2007; 119(19-20):593-6.
- Termanini B, Gibril F, Reynolds JC, Doppman JL, Chen CC, Stewart CA, et al. Value of somatostatin receptor scintigraphy: a prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997;112(2):335-47.
- Jimenez Londoño GA, García Vicente AM, Soriano Castrejon AM, Gómez López OV, Palomar Muñoz A, Vega Caicedo CH, et al. Role of 99mTc-HYNIC-Tyr3-octreotide scintigraphy in neuroendocrine tumors based on localization of the primary tumor. Minerva Endocrinol. 2016;41:10-18.
- Povoski SP, Neff RL, Mojzisik CM, O'Malley DM, Hinkle GH, Hall NC, et al. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol. 2009; 7:11.
- Kaur S, Venktaraman G, Jain M, Senapati S, Garg PK, Batra SK. Recent trends in antibody-based oncologic imaging. Cancer Lett. 2012;315(2):97-111.
- Wu AM. Engineered antibodies for molecular imaging of cancer. Methods. 2014;65(1):139–147.
- Stephen P. Povoski, Cathy M. Mojzisik, and Brandon J Sullivan. Radioimmunoguided Surgery: Intraoperative Radioimmunodetection for the Radioguided Localization and Resection of Tumors. In K. Herrmann, O. E. Nieweg, and S.P. Povoski (Eds.), Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016. 371-417.
- Povoski SP, Hall NC, Murrey DA Jr, Sharp DS, Hitchcock CL, Mojzisik CM, et al. Multimodal imaging and detection strategy with 124 I-labeled chimeric monoclonal antibody cg250 for accurate localization and confirmation of extent of disease during laparoscopic and open surgical resection of clear cell renal cell carcinoma. Surgical Innovation. 2013;20(1):59-69.
- Stephen P. Povoski. The History of Radioguided Surgery: Early Historical Milestones and the Development of Later Innovative Clinical Applications. In K. Herrmann, O. E. Nieweg, and S.P. Povoski (Eds.), Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016. 3-12.
- Andrea V. Barrio and Hiram S. Cody III. Radioguided Sentinel Lymph Node Mapping and Biopsy in Breast Cancer. In K. Herrmann, O. E. Nieweg, and S.P. Povoski (Eds.), Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016. 115-123.
- Sun D, Bloomston M, Hinkle G, Al-Saif OH, Hall NC, Povoski SP, et al. Radioimmunoguided surgery (RIGS), PET/CT image-guided surgery, and fluorescence image-guided surgery: past, present, and future. J Surg Oncol. 2007; 96:297-308.
- Daan Hellingman, Sergi Vidal-Sicart. The Use of Intraoperative Small and Large Field of View Gamma Cameras for Radioguided Surgery. In K. Herrmann, O. E. Nieweg, and S.P. Povoski (Eds.), Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice (pages 35-56). Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016.
- Hall NC, Plews RL, Agrawal A, Povoski SP, Wright CL, Zhang J, et al. Intraoperative scintigraphy using a large field-of-view portable gamma camera for primary hyperparathyroidism: initial experience. BioMed Res Int. 2015; 2015:930575.
- Hall NC, Nichols SD, Povoski SP, James IA, Wright CL, Harris R, et al. Intraoperative Use of a Portable Large Field of View Gamma Camera and Handheld Gamma Detection Probe for Radioguided Localization and Prediction of Complete Surgical Resection of Gastrinoma: Proof of Concept. J Am Coll Surg. 2015; 221:300-308.
- Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
- Julien S, Videira PA, Delannoy P. Sialyl-Tn in cancer: How did we miss the target? Biolmolecules. 2012; 2(4):435-466.
- Molinolo A, Simpson JF, Thor A, Schlom J. Enhanced tumor binding using immunohistochemical analyses by second generation anti-Tumor-associated Glycoprotein 72 monoclonal antibodies versus monoclonal antibody B72.3 in human tissue. Cancer Res, 1990; 50:1291-1298.
- Kuroki M, Fernsten PD, Wunderlich D, Colcher D, Simpson JF, Poole DJ, et al. Serological mapping of the TAG-72 tumor-associated antigen using 19 distinct monoclonal antibodies. Cancer Res. 1990; 50(16):4872-9.
- Colcher D, Hand PH, Nuti M, Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc Nati Acad Sci. 1981;78:3199-203.
- Sheer DG, Schlom J, Cooper HL. Purification and composition of the human tumor-associated glycoprotein (tag-72) defined by monoclonal antibodies CC49 and B72.3. Cancer Res. 1998;48(23):6811-8.
- Muraro R, Kuroki M, Wunderlich D, Poole DJ, Colcher D, Thor A, et al. Generation and characterization of B72.3 second generation monoclonal antibodies reactive with tumor-associated glycoprotein 72 antigen. Cancer Res. 1988; 48(16):4588-96.
- Sosolik RC, Hitchcock CL, Becker WJ. Heterophilic antibodies produce spuriously elevated CK-MB concentrations in a selected patient population. Am J Clin Pathol. 1997;107(5):506-510.
- Agnese DM, Abdessalam SF, Burak WE Jr, Arnold MW, Soble D, Hinkle GH, et al . Pilot study using a humanized CC49 monoclonal antibody (HuCC49DeltaCH2) to localize recurrent colorectal carcinoma. Ann Surg Oncol. 2004;11(2):197-202.
- Fang L, Holford NH, Hinkle G, Cao X, Xiao JJ, Bloomston M, et al. Population pharmacokinetics of humanized monoclonal antibody HuCC49DCH2 and murine antibody CC49 in colorectal cancer patients. J Clin Pharmacol 2007;47(2):227–237.
- Arnold MW, Hitchcock CL, Young DC, Burak WE Jr, Bertsch DJ, Martin EW Jr. Intra-abdominal patterns of disease dissemination in colorectal cancer identified using radioimmunoguided surgery. Dis Colon Rectum. 1996;39(5):509-13.
- Burak WE Jr, Schneebaum S, Kim JA, Arnold MW, Hinkle G, Berens A, et al. Pilot study evaluating the intraoperative localization of radiolabeled monoclonal antibody CC83 in patients with metastatic colorectal carcinoma. Surgery. 1995;118(1):103-108.
- Arnold MW, Schneebaum S, Berens A, Petty L, Mojzisik C, Hinkle G, et al. Intraoperative detection of colorectal cancer with radioimmunoguided surgery and CC49, a second-generation monoclonal antibody. Ann Surg. 1992; 216(6):627-32.
- Arnold MW, Schneebaum S, Berens A, Mojzisik C, Hinkle G, Martin EW Jr. Radioimmunoguided surgery challenges traditional decision making in patients with primary colorectal cancer. Surgery. 1992;112(4):624-630.
- Haddad R, Avital S, Troitsa A, Chen J, Baratz M, Brazovsky E, et al. Benefits of radioimmunoguided surgery for pelvic recurrence. Eur J Surg Oncol. 2001; 27:298-301.
- Schneebaum S, Troitsa A, Haddad R, Avital S, Kashtan H, Baratz M, et al. Immunoguided lymph node dissection in colorectal cancer: a new challenge? World J Surg. 2001; 25(12):1495-1499.
- Avital S, Haddad R, Troitsa A, Kashtan H, Brazovsky E, Gitstein G, et al. Radioimmunoguided surgery for recurrent colorectal cancer manifested by isolated CEA elevation. Cancer. 2000;89(8):1692-1698.
- Nieroda CA, Mojzisik C, Sardi A, Ferrara PJ, Hinkle G, Thurston MO, et al. Radioimmunoguided surgery in primary colon cancer. Cancer Detect Prev. 1990;14(6):651-656.
- Percivale P, Bertoglio S, Meszaros P, Schenone F, Gipponi M, Moresco L, et al. Radioimmunoguided surgery with different iodine-125 radiolabeled monoclonal antibodies in recurrent colorectal cancer. Semin Surg Oncol. 1998;15(4):231-234.
- Sickle-Santanello BJ, O’Dwyer PJ, Mojzisk C, Tuttle SE, Hinkle GH, Rousseau M, et al. radioimmunoguided surgery using the monoclonal antibody B72.3 in colorectal tumors. Dis Colon Rectum. 1987;30(10):761-764.
- Bertsch DJ, Burak WE Jr, Young DC, Arnold MW, Martin EW Jr. Radioimmunoguided surgery for colorectal cancer. Ann Surg Oncol. 1996;3:310-6.
- Povoski SP, Hatzaras IS, Mojzisik CM, Arnold MW, Hinkle GH, Hitchcock CL, et al. Antigen-directed cancer surgery for primary colorectal cancer: 15-year survival analysis. Ann Surg Oncol. 2012; 19:131-138.
- Bethesda. SEER Cancer Statistics Factsheets: Colon and Rectum Cancer. National Cancer Institute.
- Cornelius EA, West AB. False tumor-positive lymph nodes in radioimmunodiagnosis and radioimmunoguided surgery: etiologic mechanisms. J Surg Oncol. 1996; 63:23-35.
- Schneebaum S, Arnold MW, Houchens DP, Greenson JK, Cote RJ, Hitchcock CL, et al. The significance of intraoperative periportal lymph node metastasis identification in patients with colorectal carcinoma. Cancer. 1995; 75:2809-17.
- Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW Jr. Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer. 1994;73(3):563-9.
- Cote RJ, Houchens DP, Hitchcock CL, Saad AD, Nines RG, Greenson JK, et al. Intraoperative detection of occult colon cancer micrometastases using 125 I-radiolabled monoclonal antibody CC49. Cancer. 1996; 77:613-620.
- Hitchcock CL, Sampsel J, Young DC, Martin E Jr, Arnold MW. Limitations with light microscopy in the detection of colorectal cancer cells. Dis Colon Rectum. 1999; 42:1046-1052.
- Martinez DA, Barbera-Guillem E, LaValle GJ, Martin, EW Jr. Radioimmunoguided surgery for gastrointestinal malignancies: an analysis of 14 years of clinical experience. Cancer Control. 1997; 4:505-516.
- Hitchcock CL, Arnold MW, Young DC, Schneebaum S, Martin EW Jr, Loy TS. TAG-72 expression in lymph nodes and RIGS. Dis Colon Rectum. 1996; 39(4):473-475.
- Stephen P. Povoski, Douglas A. Murrey Jr, Nathan C. Hall, 18F-FDG-Directed Surgery and 18F-FDG-Directed Interventional. In K. Herrmann, O. E. Nieweg, and S.P. Povoski (Eds.), Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016; 421-445.