Short Communication
Arachidonate-5-Lipoxygenase (ALOX5): A Master Regulator for Prostate Cancer Cell Survival
Jagadananda Ghosh*
Departments of Urology, The Josephine Ford Cancer Center, Henry Ford Health System, Detroit, Michigan, USA
*Corresponding author: Jagadananda Ghosh, Departments of Urology, The Josephine Ford Cancer Center, Henry Ford Health System, Detroit, Michigan, USA
Published: 04 May, 2017
Cite this article as: Ghosh J. Arachidonate-5-Lipoxygenase
(ALOX5): A Master Regulator for
Prostate Cancer Cell Survival. Clin
Oncol. 2017; 2: 1277.
Short Communication
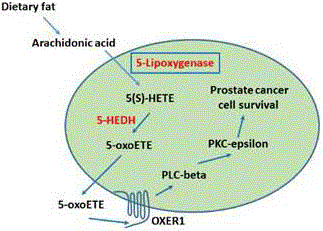
Metabolic reprogramming has been recognized as a hallmark of cancer. Cancer cells grow and divide much more rapidly than normal cells, meaning that they have much higher demand for nutrients and oxygen. Cancer cells in general show a strong avidity for lipids which they satisfy by increasing uptake and/or by de novo synthesis. Excess lipids (fatty acids, cholesterols, etc) are stored in cancer cells as Lipid Droplets (LD) which is a common feature of more aggressive cancer cells, and LD-rich cancer cells are more resistant to chemotherapy. In general, high-fat “Western-style” diets have repeatedly been found to be associated with increased incidence of clinical cancers of the prostate, colon, breast, pancreas, and esophagus [1]. Though a possible connection between fat and various types of cancer has been recognized for quite some time, molecular understanding about how dietary fat may work to promote development and/or promotion of cancer is still at its infancy. The role of lipids in cancer is heightened by the fact that fatty acids play a dual role in cancer promotion: as energy producer via the beta-oxidation pathway, and [2] by making metabolites which deliver powerful signals to promote cancer cell growth and proliferation, as well as to control apoptotic cell death. Important changes in lipid composition, such as Saturated Fatty Acids (SFA) vs. Unsaturated Fatty Acids (UFA), or Mono-Unsaturated Fatty Acid (MUFA) vs. Poly-Unsaturated Fatty Acids (PUFA), as well as their relative abundance severely alter membrane fluidity and protein dynamics. Interestingly, in addition to the structural role, lipids orchestrate a variety of signal transduction cascades. They can also be broken down into bioactive lipid-mediators, which in turn may regulate a variety of carcinogenic processes, including cell growth, cell migration, invasion, and metastatic colonization [2]. Metabolism of arachidonic acid is probably the prime example of how dietary fat/fatty acids can play a role in promoting cancer. Arachidonic acid is an omega-6 polyunsaturated fatty acid abundant in our diets and is metabolized via a number of pathways, such as cyclooxygenase(s), lipoxygenase (s), and epoxygenase [2,3]. Metabolic products of arachidonic acid are called by the common term eicosanoids, which have been documented to play a multitude of cellular activities needed for various functions in cancer cells. One notable example of an important role of eicosanoids in cancer is the regulation of cancer cell survival by a group of arachidonic acid metabolites which are generated via the 5-lipoxygenase pathway. Both leukotrienes and the hydroxyeicosatetraenoids (HETEs) are products of 5-lipoxygenase activity. However, it appears that the HETE-series of metabolites play critical roles in the survival of prostate (and possibly other types of) cancer cells. So, the picture has unfolded as, that arachidonic acid is metabolized via 5-lipoxygenase to generate 5(S)-HETE series of eicosanoids which are essential for prostate cancer cells to survive, and that inhibition of 5-lipoxygenase by specific chemical inhibitors or siRNAs kill prostate cancer cells via induction of apoptosis [4-9]. Thus, 5-lipoxygenase has emerged as a new, promising target to kill prostate cancer cells via triggering of apoptotic cell death. How 5-lipoxygenase metabolites exert survival-promoting effects in prostate cancer cells was the topic of great interest in the last decade. Surprisingly, it was found that inhibition of 5-lipoxygenase does not inhibit Akt or ERKs which are known for their roles in cell survival. This finding brought a new twist in the biology of cancer cell survival because Akt and ERKs are well-characterized to promote cell viability through defined mechanisms in a variety of cells. After intense and elaborate studies it was found that it is the PKC-epsilon which mediates the survival-promoting effects of 5-lipoxygenase in prostate cancer cells [8,9]. Inhibition of 5-lipoxygenase rapidly inhibits the activity of PKC-epsilon (which is continuously active in prostate cancer cells) and induces apoptosis in prostate cancer cells via activation of c-Jun N-terminal Kinase (c-JNK) [7]. It was also found that the active 5-lipoxygenase metabolite, 5-oxoETE (a dehydrogenated derive of 5(S)-HETE), signals via its cognate receptor, called OXER1, which is a member of the seven-transmembrane G proteincoupled receptor (GPCR) family. This receptor has been cloned from human PC3 prostate cancer cells and sequenced which confirmed the expression of OXER1 in any kind of cancer cells for the first time [10]. Through a series of experiments, it was demonstrated that OXER1 is connected to the activation of PKC-epsilon via phospholipase C-beta (PLC-beta) which generates diacylglycerol (DAG) to activate PKC-epsilon [11]. Recently, it was found that 5-lipoxygenase activity plays a role in the regulation of c-Myc oncogene in prostate cancer cells [12]. C-Myc has been characterized to be a strong promoter of the regulation of prostate cancer cell viability, proliferation, and prevention of apoptosis. Thus, the feat of arachidonic acid metabolites to exert survival signals in prostate cancer cells is uniquely positioned so that the prostate cancer cells can harvest the supply of metabolic gasoline from high dietary fats, especially animal fats which are amply present in red meat, egg yolk, and dairy products. Based on the currently available experimental data the following model emerges which summarizes the main points involved in the regulation of prostate cancer cell survival by arachidonate-5-lipoxygenase (Figure 1).
Figure 1
Figure 1
Arachidonic acid from diet enters the cell and is converted to 5(S)-
hydroxyeicosatetraenoic acid [5(S)-HETE] by the action of 5-lipoxygenase.
Then, 5(S)-HETE is converted by its dehydrogenase (5-HEDH) to 5-oxoETE
which binds with its cognate GPCR (OXER1) on the plasma membrane.
ActivatedOXER1 in turn activates PLC-beta that generates diacylglycerol
and activates PKC-epsilon. PKC-epsilon is a strong cancer-promoter, and
regulates both extrinsic and intrinsic mechanisms of apoptosis to promote
survival of prostate cancer cells.
References
- Kushi L, Giovannucci E. Dietary fat and cancer. Am J Med. 2002;113;9B:63S-70S.
- Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181-93.
- Ghosh, J. Targeting 5-lipoxygenase for prevention and treatment of cancer. Current Enzyme Inhibition. 2008;4:18-28.
- Ghosh J, Myers CE. Arachidonic Acid Stimulates Prostate Cancer Cell Growth: Critical Role of 5-Lipoxygenase. Biochem Biophys. Res Commun. 1997;235:418-423.
- Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. USA. 1998;95:13182-13187.
- Ghosh J, Myers CE. Central role of arachidonate 5-lipoxygenase in the regulation of cell growth and apoptosis in human prostate cancer cells. Adv Exp Med Biol. 1999;469:577-82.
- Ghosh J. Inhibition of arachidonate 5-lipoxygenase triggers prostate cancer cell death through rapid activation of c-Jun N-terminal kinase. Biochem. Biophys. 2003;307:342-349.
- Sarveswaran S, Myers C E, Ghosh J. MK591, a leukotriene biosynthesis inhibitor, induces apoptosis in prostate cancer cells: synergistic action with LY294002, an inhibitor of phosphatidylinositol 3'-kinase. Cancer Lett.2010;291:167-176.
- Sarveswaran S, Thamilselvan V, Brodie C, Ghosh J. Inhibition of 5-lipoxygenase triggers apoptosis in prostate cancer cells via down-regulation of protein kinase C-epsilon. Biochim. Biophys. Acta. 2011;1813:2108-2117.
- Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Commun. 2006;339(1):93-8.
- Sarveswaran S, Ghosh J. OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells. Cancer Lett. 2013; 336:185-195.
- Sarveswaran S, Chakraborty D, Chitale D, Sears R, Ghosh J. Inhibition of 5-Lipoxygenase selectively triggers disruption of c-Myc signaling in prostate cancer cells. J Biol Chem. 2015;290:4994-4506.