Case Report
Two Patients with Advance-Stage Renal Cell Carcinoma Developed Sunitinib-Related Skin Toxicity
Mehmet Cetinkaya1*, Ozgur Tanriverdi2, Hasan Deliktas1, Asude Kara3, Yelda Dere4 and Hayrettin Sahin1
1Department of Urology, School of Medicine, Mugla Sitki Kocman University, Mugla, Turkey
2Department of Medical Oncology, School of Medicine, Mugla Sitki Kocman University, Mugla, Turkey
3Department of Dermatology, Mugla Sitki Kocman University Training and Research Hospital, Mugla, Turkey
4Department of Pathology, School of Medicine, Mugla Sitki Kocman University, Mugla, Turkey
*Corresponding author: Mehmet Cetinkaya, Department of Urology, Mugla Sitki Kocman University, Mugla, 48000, Turkey
Published: 26 Apr, 2017
Cite this article as: Cetinkaya M, Tanriverdi O, Deliktas
H, Kara A, Dere Y, Sahin H. Two
Patients with Advance-Stage Renal
Cell Carcinoma Developed Sunitinib-
Related Skin Toxicity. Clin Oncol. 2017;
2: 1269.
Abstract
With the current treatment modalities like molecular targeted therapy, general survival rates have
increased in patients with advanced-stage renal cell carcinoma. Sunitinib maleate is one of these
drugs and its most frequently side effects are fatigue, diarrhea, hypertension, stomatitis, and hair
hypo-pigmentation. Additionally, various dermatological side-effects may also be seen such as handfoot
syndrome, a yellowish color change to the face, splinter hemorrhage, erythematous reactions
on the trunk, facial edema, facial erythematous changes, alopecia, acneiform rash on the face and
dysesthesia in the scalp. Although Sunitinib maleate-related scrotal skin toxicity is extremely rarely
seen, it should not be forgotten that this side-effect can be easily managed. Herein, we report two
cases with Sunitinib maleate-related scrotal skin toxicity, which were diagnosed with advancedstage
renal cell carcinoma in this case report.
Keywords: Sunitinib; Kidney cancer; Scrotal cutaneous toxicity; Molecular targeted therapy
Introduction
With molecular targeted treatment, overall survival rates have been recently increased in patients
with advanced-stage Renal Cell Carcinoma (RCC) [1]. Sunitinib maleate, an orally tyrosine kinase
inhibitor, is one of these targeted molecules which have small molecular weight [1]. It is widely used
in the treatment of patients with advanced-stage RCC, metastatic neuroendocrine carcinoma, and
gastrointestinal stromal tumors [1,2].
Most frequently side-effects in patients using sunitinib maleate are fatigue, diarrhea,
hypertension, stomatitis, and hair hypo-pigmentation [1-3]. Additionally, various dermatological
side-effects may also be seen such as hand-foot syndrome, a yellowish color change to the face,
splinter hemorrhage, erythematous reactions on the trunk, facial edema, facial erythematous
changes, alopecia, acneiform rash on the face and dysesthesia in the scalp [1-3]. However, sunitinib
maleate-related scrotal skin toxicity is rarely seen [3,4].
Herein, we report two cases with sunitinib maleate-related scrotal skin toxicity, which were
diagnosed with advanced-stage renal cell carcinoma in this paper.
Case Presentation
Case 1
A 72-year old male, who have not previous disease other than controlled hypertension,
presented with complaints of increasingly worsening shortness of breath and cough. A solid mass
in the left kidney with showing heterogeneous contrast, its size was approximately 7cm x 8cm, was
determined in Computerized Tomography (CT) as well as multiple lymph nodes in mediastinum
and bilateral multiple nodules in pulmonary parenchyma. Moreover, he has widespread bone
metastases in thoracic as well as lumbar vertebrae. Then biopsy was performed from the mass in the
left kidney and it was diagnosed as a clear cell renal carcinoma.
After consultation with the Medical Oncology Department, the patient was started on treatment
of interferon 2α. In the evaluation made after 3 months of treatment, progression of the disease
was determined. Treatment was then started of 50mg Sunitinib maleate for 4 weeks every 6 weeks.
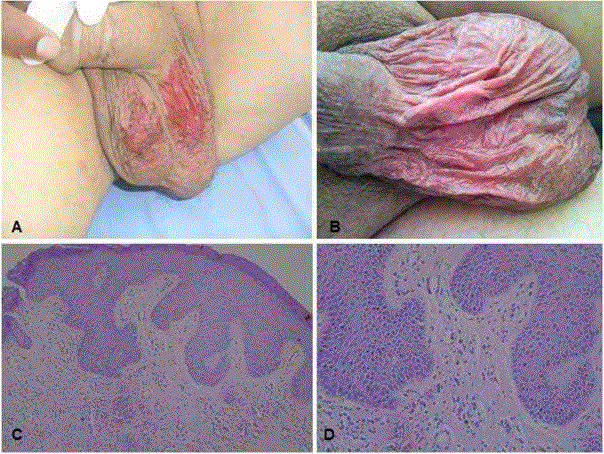
On the 24th day of treatment, the patient presented with a skin reaction on the scrotum, which
was clearly defined with scattered desquamation areas in the form
of erythematous atrophic plaque (Figure A). A biopsy was taken
from the lesion. The Sunitinib maleate treatment was halted and
within 10 days the lesion completely recovered and the biopsy result
was evaluated as drug reaction. The patient was re-started on the
same treatment scheme and on the 16th day similar scrotal lesions
developed and again when the Sunitinib treatment was halted, the
findings recovered. The patient was started on a treatment program
of continuous Sunitinib maleate at 25mg/day and apart from mild
scrotal erythematous plaques which lasted approximately 1 week, no
other skin reaction was observed. After approximately 7 treatment
cycles, progression developed in pulmonary metastasis and with a
worsening of general status the patient was lost 11 months after the
diagnosis.
Case 2
A 77-year old male, who was previously healthy, presented with
complaints of back and side pain which had been increasing for
approximately 2 months. On the examination with CT, a lobular
contoured solid mass in the right kidney with showing heterogeneous
contrast, its size was 77mm x 59mm and bilateral multiple nodules
in pulmonary parenchyma were determined as well as widespread
bone metastases in scintigraphic examination. With right-side
nephrectomy, a diagnosis was made of Grade T1 clear cell carcinoma
limited to within the renal capsule, 6.5cm in size, of nuclear
degree Fuhrman IV which had general necrosis and mitosis. Then
interferon 2α treatment was initiated by the Oncology Department.
In the second month of treatment, intolerance and progression of
pulmonary metastasis were observed and therefore the treatment
was continued with 50mg/day Sunitinib maleate for 4 weeks every
6 weeks. However, similar scrotal lesions, which were clearly defined
with scattered desquamation areas in the form of erythematous
atrophic plaque, developed on the 14th day of treatment (Figure B).
The Sunitinib maleate treatment was halted and within 10 days the
lesion completely recovered and the biopsy result was evaluated as
drug reaction. Treatment of 25mg/day continuous Sunitinib maleate
was started and no skin reaction was observed. The patient then
transferred to another centre for follow-up and as the patient could
not be contacted; there is no further information available on the
clinical course.
On dermatological examination, widespread erythematous
atrophic plaques with scattered desquamation were observed on the
scrotum (Figure A and B).
Histopathological examination showed interface dermatitis
characterized by vacuolar degeneration in the basal layer and
dyskeratotic cells with perivascular mononuclear inflammatory cells
in the dermis and these findings were reported as consistent with
drug induced dermatitis (Figure C and D).
Figure
Figure
Macroscopic images and microscopic examination of sunitinib-related
skin toxicity, A-B: Macroscopic image of Case 1 and Case 2, respectively
showing live erythematous atrophic plaque with scattered desquamated
areas clearly limited to the scrotum. C-D: Scrotum skin biopsy microscopic
examination (HEx100, HEx200): interface dermatitis findings characterized
by vacuolar degeneration in the basal layer and dyskeratotic cells with
perivascular mononuclear inflammatory cells to the dermis, consistent with
drug induced dermatitis.
Discussion and Conclusion
Although various dermatological side-effects have been seen
in patients using Sunitinib maleate, prevalence of its skin toxicity
has been reported as nearly <15% in previous studies. Moreover,
Sunitinib maleate-related scrotal skin toxicity is extremely rare [1-4].
Sunitinib maleate-related scrotal skin toxicity was first described
by Billemont “et al”. in 2008 [3]. In a total of 40 RCC patients
treated with Sunitinib maleate, it was reported in only 5 (12%)
patients that scrotal erythematous lesion developed accompanying
desquamation. Subsequently, a case of Sunitinib-related recurrent
scrotal hemangiomas was reported by Tonini “et al”. in 2010 [5].
Although the mechanism of scrotal cutaneous toxicity has not
been clarified, Billemont “et al”. [3] hypothesized that the underlying
mechanism of this toxicity could be the anti-angiogenic effect of
Sunitinib maleate. It is also thought that hypoxia-inducible factor-
1α (HIF-1α) and Vascular Endothelial Growth Factor (VEGF) play
an important role in Sunitinib-related cutaneous toxicity. This view
has been supported in studies showing an in vitro increase in plasma
VEGF levels after treatment with Sunitinib maleate in patients with
RCC [3]. Moreover, in patients with psoriasis, it has been shown that
HIF-1α was strongly unregulated and at the same time angiogenesis
was induced by VEGF [6]. As a result, increased limited oxygen
diffusion within the tissues in both normal and tumor, which could
be explained by paradoxical hypoxia, could cause an anti-angiogenic
process.
In the most recent article on Sunitinib maleate-related scrotal
skin toxicity by Chou “et al.” [7], Sunitinib maleate-related scrotal
cutaneous toxicity was confirmed by biopsy as well as indicated that
strongly stained with VEGF by immunohistochemically examination
was determined in the endothelial cells of the dermal blood vessels
in a 74-year old male patient with RCC. This was the first paper to
show the relationship between VEGF and Sunitinib maleate-related
scrotal cutaneous side-effect. Chou “et al.” [7] showed that there was
no staining with VEGF in normal scrotal tissue taken from a healthy
male and thus concluded that VEGF played a significant role in
Sunitinib maleate-related scrotal skin toxicity [7].
Although cases presented in literature differ from each other
in respect of Sunitinib dose and application schedule, the scrotal
cutaneous side-effect was observed to develop at 7-14 days after
starting the medication [3,4,6]. This can suggest that there is no
significant relationship between medication dosage and scrotal
cutaneous toxicity. In our cases, the side-effects were observed after
exposure to the drug, which is consistent with the data in literature.
Similarly it has been reported that within a week of stopping the
Sunitinib treatment, cutaneous findings recovered. In the current
cases, it was also seen that the cutaneous findings recovered one week
after halting the Sunitinib treatment.
The conclusion can be reached that the Sunitinib-maleate related
cutaneous side-effect recovers after stopping the medication and this
side-effect can be easily managed with dose modification. Therefore,
that scrotal cutaneous toxicity could develop but this is a manageable
side-effect must be explained to patients receiving Sunitinib-maleate
treatment.
In conclusion, although rare in the follow-up of patients receiving
Sunitinib maleate treatment, scrotal cutaneous toxicity should not be
forgotten as one of the dermatological side-effects which may be seen.
When it is taken into account that a significant increase in survival
rates is achieved, this side-effect can be considered not to play a
significant role in discarding the medication.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Ara M, Pastushenko E. Antiangiogenic agents and the skin: cutaneous adverse effects of sorafenib, sunitinib, and bevacizumab. Actas Dermosifiliogr. 2014;105(10):900-12.
- Rosenbaum SE, Wu S, Newman MA, West DP, Kuzel T, Lacouture ME. Dermatological reactions to the multitargeted tyrosine kinase inhibitor sunitinib. Support care cancer. 2008;16(6):557-66.
- Billemont B, Barete S, Rixe O. Scrotal cutaneous side effects of sunitinib. N Engl J Med. 2008;359(9):975-6.
- Iacovelli R, Mancini ML, Risi E, Palazzo A, Cortesi E. Genital and inguinal cutaneous toxicity in male and female patients treated with sunitinib. Int J Dermatol. 2012;51(2):221-2.
- Tonini G, Intagliata S, Cagli B, Segreto F, Perrone G, Onetti Muda A, et al. Recurrent scrotal hemangiomas during treatment with sunitinib. J Clin Oncol. 2010;28(35):e737-8.
- Simonetti O, Lucarini G, Goteri G, Zizzi A, Biagini G, Lo Muzio L, et al. VEGF is likely a key factor in the link between inflammation and angiogenesis in psoriasis: results of an immunohistochemical study. Int J Immunopathol Pharmacol. 2006;19(4):751-60.
- Chou CY, Wang KH, Lin YH, Lin YT, Tsai HH. Sunitinib-induced scrotal cutaneous side-effect. J Dermatol. 2013;40(1):67-8.