Research Article
Multi-Professional Follow-Up Programmes are Needed to Address Psychosocial, Neurocognitive and Educational Issues in Children with Brain Tumours
I van’t Hooft1,3*, A Lindahl Norberg1,2, A Björklund4, M Lönnerblad3,5 and B Strömberg4,6
1Department of Women’s and Children’s Health, Childhood Cancer Research Unit, Karolinska Institutet, Stockholm, Sweden
2Department of Public Health and Caring Sciences, Clinical Psychology in Health Care, Uppsala University, Uppsala, Sweden
3Department of Women’s and Children’s Health, Neuropaediatric Unit, Astrid Lindgren Children’s Hospital, Karolinska University Hospital, Stockholm, Sweden
4Department of Women’s and Children’s Health, Uppsala University Children’s Hospital, Uppsala, Sweden
5Department of Women’s and Children’s Health, National Agency for Special Needs Education and Schools, Stockholm, Sweden
6Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden
*Corresponding author: Ingrid van’t Hooft, Department of Women’s and Children’s Health, Astrid Lindgren Children’s Hospital, Karolinska University Hospital, Stockholm, Sweden
Published: 24 Mar, 2017
Cite this article as: van’t Hooft I, Lindahl Norberg A,
Björklund A, Lönnerblad M, Strömberg
B. Multi-Professional Follow-Up
Programmes are Needed to Address
Psychosocial, Neurocognitive and
Educational Issues in Children with
Brain Tumours. Clin Oncol. 2017; 2:
1248.
Abstract
Aim: The aim of this study was to coordinate the structured psychosocial, neurocognitive and
educational follow up of children treated for brain tumours with the medical protocol and apply
the model in two Swedish healthcare regions.
Methods: We invited all children living in the two regions, who had been diagnosed with a brain
tumour from 1 October 2010 through to 30 June 2012, to participate along with their parents. The
follow-up programme evaluated the emotional status of the parents and patients and assessed the
children’s general cognitive level, working memory, speed of performance, executive functions and
academic achievement from diagnosis through to adult care.
Results: During the study period, 61 children up to the age of 17.1 years were diagnosed with a
brain tumour, but 18 of these were excluded for various reasons. The majority of the mothers (70%)
displayed significantly poor emotional status, as did 34% of the fathers and 21% of the children. The
majority of the children (57%) also showed poor neurocognitive performance and needed special
adaptations at school (66%).
Conclusion: Our findings indicate the need for coordinated, multi-professional follow-up
programmes, well anchored in the healthcare organisation, for children diagnosed with brain
tumours.
Keywords: Multi-professional care; Neurocognitive performance, Paediatric brain tumours, Poor emotional status, Special needs
Key Notes
• This study focused on 43 children who had been diagnosed with a brain tumour and found
that most of them (57%) showed poor neurocognitive performance and needed special
adaptations at school (66%).
• The majority of the mothers (70%) displayed significantly poor emotional status, as did 34%
of the fathers and 21% of the children.
• The findings indicate the need for coordinated, multi-professional follow-up programmes
for children diagnosed with brain tumours.
Introduction
Brain tumours account for almost 30% of all malignant disorders in childhood and are the second most common tumour type in children [1]. These days, 70% of children with brain tumours who live in Europe survive for more than five years after diagnosis and most reach adulthood [1,2]. However, many of the survivors experience various sequelae, which are caused by the tumour itself or by the surgery, irradiation and, or, chemotherapy they receive to treat the tumour [3-5]. Despite radically improved survival rates for most paediatric cancers, patients and families display a number of psychological reactions, including anxiety, when they are given the diagnosis [6]. The outcome is uncertain throughout the treatment period and the treatment itself entails prolonged stress, which particularly affects parents [6,7]. Moreover, parents have been known to display feeling of distress and abandonment years after their child successfully completes their treatment [8,9]. Therefore, psychosocial monitoring of the entire family, starting at the point of diagnosis, has been suggested [6]. As children who survive brain tumours face the risk of late neurocognitive deficits [10-12], as well as endocrine [4] and neurological [3] dysfunctions, the psychological burden on the children, parents and the whole family is great. The child’s cognitive problems must also be recognised early and taken into consideration when planning the child’s schooling [13,14]. Furthermore, children without problems must be identified in order to avoid subjecting them to lengthy neurocognitive investigations. In order to make this screening possible, the test methods that are adopted have to be reliable, valid, and easily used in routine clinical settings. This prospective project took place in the Swedish healthcare regions of Stockholm and Uppsala-Örebro. Its aim was to organise the psychosocial, educational and neurocognitive follow up in such a way that it could be used for all children diagnosed with a brain tumour, as well as their parents. We also wanted to coordinate this support with the medical follow-up programme, and adjust it to the clinical setting, so that all children and parents underwent appropriate investigations and received the help they needed.
Subjects and Methods
Sweden is divided into six healthcare regions that vary in
geographical size and number of inhabitants. The Stockholm region
is a typical urban region, where roughly 2,250,000 people inhabit a
fairly small geographical area of 9,660 km², while the Uppsala-Örebro
region is a mainly rural district, with an area of 75,800 km² and
1,740,000 inhabitants.
Subjects
We approached families living in the Stockholm and UppsalaÖrebro
healthcare regions who had a child under 18 years that had
been diagnosed with a brain tumour between 1 October 2010 and
30 June 2012 and invited them to take part in this prospective study.
Participants were recruited by the consultant nurse for brain tumours
or by the responsible paediatrician at either the Astrid Lindgren
Children’s Hospital or the Uppsala University Children’s Hospital.
The inclusion criteria were that child had been diagnosed with a
brain tumour, irrespective of the location and grade of malignancy,
together with their age, place of residence and the family’s ability to
speak Swedish. Oral and written information was given to all parents
and written consent was received from all families that were included.
The study was approved by the Regional Ethics Review Board of
Uppsala University. During the study period, 61 children from birth
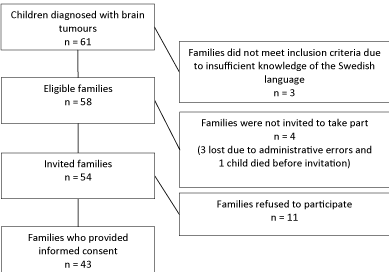
to 17.1 years were diagnosed with brain tumours. Of those three
were excluded because the family did not speak sufficient Swedish,
11 families refused to participate, one child died before being invited
and three were not invited because of an administration error (Figure
1). Thus, the study group comprised the families of 43 children
diagnosed with brain tumours, 74% of the 58 eligible to take part.
The characteristics of the children who were included are presented in Table 1. They included the ten children who died during the study period.
In the study group, four children were infants under one year
of age at diagnosis, 20 children were 1-5.9 years old and 19 were
6-17.1 years old. In Sweden, children start preschool at six years
of age and it is compulsory for them to attend school from the age
of seven to the age of 19. The majority of the children we studied
attended elementary or middle school. There were 38 children who
lived with both biological parents and five children whose parents
were no longer together. There were 34 children with siblings and the
remaining nine were an only child.
Children in the Uppsala-Örebro region who had just undergone
an operation stayed at the regional hospital for two to three weeks
and were followed up at their local paediatric clinics, while children
who received surgery in the Stockholm region were followed up at the
outpatient clinic in Stockholm. If they were treated with radiotherapy
for six to eight weeks following surgery they received this at the hospitals
in Stockholm or Uppsala. Most of the families living in Stockholm
only needed to pay day visits to the hospital during radiotherapy, but
most of the children living in Uppsala-Örebro had to stay with one or
both parents in a hotel or apartment close to the university hospital
in Uppsala, because of the distance from their home. This meant that
they were separated other family members and friends during that
time. Children who needed any kind of chemotherapy or multimodal
therapy received this for up to 18 months according to the treatment.
Chemotherapy was administered at the university or local hospitals
under the supervision of the oncologists at Stockholm or Uppsala. All
children visited either the Astrid Lindgren Children’s Hospital or the
Uppsala University Children’s Hospital for an annual medical follow up.
Medical follow up
All children underwent a thorough medical examination,
including an endocrinological and neurological evaluation.
Audiometry and visual examinations were performed as necessary.
Magnetic resonance imaging scanning was also conducted,
in accordance with the medical follow-up protocol agreed by the
Swedish brain tumour group for children.
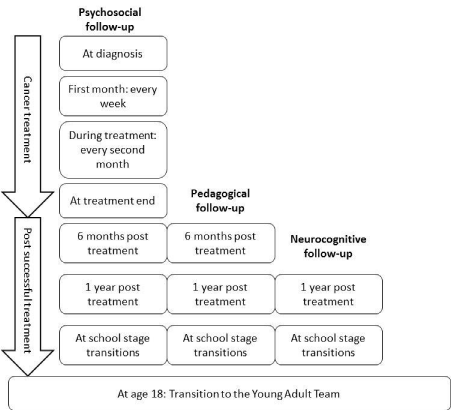
Psychosocial follow-up
The programme specified that the psychosocial follow-up should
be performed by a psychologist or a person with equal competency.
In practice, psychologists were not available and the psychosocial
assessments were carried out by social workers. The psychosocial
follow up consisted of a two-step assessment involving screening and, when needed, a more detailed assessment of the patients’ and parents’
emotional status, specifically their level of depression or anxiety,
including traumatic reactions and chronic stress or burnout. Screening
for all the study patients, mothers, and fathers followed a set schedule
(Figure 2). Those who exhibited poor emotional status at screening
were further assessed and, if necessary, referred for psychological
intervention. In addition, all patients and parents who requested
psychological intervention were given access to this, regardless of
the assessment results. Participation in the project did not affect the
families’ access to regular psychosocial services. The psychosocial
screening involved a clinical assessment of the emotional status of the
patients and parents based on observation, informal conversation,
and, if necessary, a short semi-structured interview. Screening
was conducted face-to-face or, if more convenient, by phone. The
screening was systematised using a classification tool for symptom
levels, including anxiety and depression. This ranged from zero for
a neutral mood with normal variations to five to nine for persistent
and overwhelming feelings of anxiety and persistent experiences of
severe depression. A symptom level of three or more was considered
indicative of poor emotional status warranting further assessment. At
each assessment, the parent was asked whether he or she agreed to
be called again according to the schedule (Figure 2). If participants
had symptoms in the three to nine range, further assessments took
place during a personal appointment using observation, informal
conversation and an established self-report instrument selected for
the specific type or types of emotional problem indicated by the screening.
Educational follow-up
Children who were between seven and 18 years of age were eligible
for an educational screening on two occasions during the project
period. The first was conducted six months after they finished their
cancer treatment. This included a questionnaire for the children’s
teachers regarding the prerequisites for learning - attention, memory
and information processing - as well as academic achievement and if they needed extra support at school. The second was carried out at
the hospital one year after the end of treatment by a special education
teacher and this consisted of standardised tests in reading speed
[15,16], reading comprehension [15-17] and basic arithmetic skills [18].
Neurocognitive follow-up
The neurocognitive screening battery that was used one year after
the end of treatment consisted of the Wechsler Intelligence Scale for
Children - Fourth Edition (WISC-IV) [19], which assesses general
cognitive level, verbal and perceptual functions, working memory
and performance speed, with a mean of 100 and standard deviation
of 15 for children aged between 6-18 years. Children aged 4-5.9 years
were assessed with the Wechsler Preschool and Primary Scale of
Intelligence - Third Edition (WPPSI-III) [20], which does not assess
working memory.
Executive functions were assessed using the Behavior Rating
Inventory of Executive Function (BRIEF) questionnaire for parents
and teachers [21]. This contains information about behavioural
regulation, the child’s ability to sustain working memory and to
initiate, plan, organise and monitor their own behaviour, as well as a
global executive composite. A result of more than 60 is one standard
deviation above average and an indication of poor function.
Parental satisfaction with the follow-up programme
A parental satisfaction questionnaire, designed specifically for the
study, was sent to mothers and fathers if their child was still alive
at the end of the project period. The questionnaires were completed
anonymously.
Figure 1
Table 1
Table 1
Characteristics of patients diagnosed with brain tumours in the Stockholm (St) and Uppsala-Örebro (U-Ö) healthcare regions during the project period.
Figure 2
Results
follow-up
In 37 of the 43 families that provided informed consent, at least one
family member completed an emotional status follow up on at least
one occasion. Implementing the psychosocial follow-up screening
schedule proved to be challenging. Several of the families who agreed
to participate in the project were not followed up as scheduled or the
follow up was terminated ahead of schedule. Only a few of the study
families were followed up at all the scheduled points. In addition,
two of the fathers and 18 of the patients were never followed up for
emotional status. The reasons why scheduled follow-up assessments
were not completed included referrals to a psychologist and errors in
project administration. In addition, the project-specific psychosocial
follow up was discontinued if the child died.
The clinical assessment tool identified poor emotional status at
some point, with a symptom level of three or more, for 26 of the 37
(70%) screened mothers, 12 of the 35 (34%) screened fathers and four
of the 19 (21%) screened patients.
Educational follow-up
According to the schedule, educational screening was due to take
place on 13 of the school-age children six months after treatment,
but only nine of the children’s teachers completed the screening.
Five teachers described slower processing speed, but the overall
performance with regard to attention, memory and learning was
equal to that of classmates. However, six of the children required
extra tutoring, attended a special programme or studied an adjusted
curriculum.
A special education teacher also met seven of the children at the
hospital one year after treatment for the scheduled educational followup
assessment covering reading speed, reading comprehension and
basic arithmetic skills. Only one of the seven children had results
that were equal to, or higher than, average for their age, with a mean
score of five (standard deviation 1.97) on the standard nine-point
scale in all three tests. Six of the children performed below average for
their age in reading speed, one had below average results in reading
comprehension and two had below average results in basic arithmetic
skills.
Neurocognitive follow-up
Of the 31 children between five and 18 years old, 21 were assessed
with the neurocognitive battery one year after treatment and 15 parents
and nine teachers answered the BRIEF questionnaire describing the
child’s executive performance outside a test situation. Some of the
patients did not complete the assessment because of a relapse, the
length of their treatment period or because they were too young to
be assessed. Two patients were regarded as too high functioning
for an evaluation by the responsible physician and two families in
Stockholm did not agree to the assessment taking place. In the group
of 21 children who underwent neurocognitive screening, 17 had an
average cognitive level for their age and four had a general cognitive
level below average for their age. Working memory and processing
speed were assessed for 17 of the 21 children and this showed that
three had a poor working memory and five showed poor processing
speed. In all, 12 of the 21 children who were assessed exhibited poor
neurocognitive performance in some respect. Of the 21 patients that
were assessed using the neurocognitive battery, seven had undergone
multimodal treatment, that is surgery, chemotherapy and radiation.
Four of these seven children showed a general cognitive level that was
below average for their age and low performance in both working
memory and in performance speed. The other three children who
received multimodal treatment showed an average cognitive level, but
significantly slower processing speed. Only one of the children who
received multimodal treatment displayed an average performance for
their age on all measures. Nine of the 21 children who were assessed
only received surgery and all nine of those showed an average
cognitive level. However, a further seven showed lower performance
levels in working memory and speed than expected for their age. One
child who received surgery and chemotherapy exhibited an average
intellectual level, but significantly slower performance speed. Two
children only received chemotherapy and one of those exhibited
problems with working memory.
Parents’ satisfaction with the follow-up programme
The parental satisfaction questionnaire was distributed to the 66
parents of the 33 eligible children and was completed by 34 parents.
The closed-question responses revealed that 19 parents felt that the
frequency of the psychosocial follow up was just right, 13 thought
the follow up should have been more frequent, one would have liked
less frequent follow up and one did not answer the question. When
it came to psychosocial follow up itself, 27 parents were satisfied, six
parents were dissatisfied and one did not answer the question. The
qualitative thematic content analysis of the responses to the open
ended questions revealed mixed positive and negative experiences.
The negative experiences typically focused on being abandoned in one
way or another. In the words of one parent: “We were satisfied with
the support we received during the emergency period, but afterwards,
if we had not ourselves asked for support, the support would have
been almost non-existent”. In relation to the follow-up programme,
these accounts described a number of situations in which families appeared to have been overlooked during different phases of the
schedule. A few parents commented that asking the parents to assess
the patient’s emotional status as part of the follow-up process placed
excessive responsibility on them. As one said: “The screening has
been carried out through us as parents and it is hard for us to know
how the child experienced the situation”. Furthermore, the protocol
was criticised for not including siblings in the follow up. The positive
comments conveyed an appreciation of the recurring follow up. A
number of parents stated that psychosocial follow up was essential
both during the initial phase of crisis and chaos and once treatment
had been completed and the family had returned to everyday life. As
one parent told researchers: “You also need help after the acute phase,
to be able to cope with the medical follow ups every three months and
with anxiety about the future.” At the same time, parents typically did
not realise the necessity for psychosocial support. One parent stated
that the psychosocial follow up should be “more or less compulsory,
because as a parent you think that you don’t need it”. Another stated
that “parents in crisis do not request the help they need.” Moreover,
a number of parents emphasised the importance of psychological
competence and professionalism in the encounter with families in
crisis, revealing mixed experiences. The parents were asked about
their overall satisfaction with the psychosocial follow up. One replied
that it was “much better than expected as we met an extremely skilled
person” while another stated that “in such a vulnerable position we
needed contact with a psychologist who could give more support and
guidance, but the competence and reflection were lacking”. Of the
34 parents who answered the parental satisfaction questionnaire, 21
had a child who had undergone a neurocognitive assessment and 20
of these were rather or very satisfied with that assessment. The openended
questions indicated that it was important for parents to meet
the assessment team and to receive both oral and written information
about the results. As one parent said: “It is helpful to have the results
on paper so you can understand what it means as you easily forget
the oral information”. They also stressed the importance of an
action programme and follow up at school. One parent suggested “a
mandatory meeting, perhaps two months after surgery, going through
the opportunities concerning school, rehabilitation and the future”.
Discussion
This small multi-professional project tried to meet the need for psychosocial, educational and neurocognitive follow up that children with brain tumours and their families have. We were also keen to coordinate this follow up in a practicable way with the medical protocol during treatment and afterwards. This was a feasible task, but it was not without problems. The aim of the psychosocial follow up was to monitor families through the different phases of the cancer trajectory. However, this proved difficult, mainly due to organisational and administrative factors in the two regions, which are geographically very different. Despite this, at least one family member in most families showed poor emotional status at some point and the parental satisfaction reports demonstrated that the psychosocial follow up was important, a finding that was consistent with other studies [6]. A feeling of abandonment was obvious in many families, especially when the treatment had finished, irrespective of the type of brain tumour and the treatment received. This must be taken into account when organising follow ups of this kind. Moreover, this programme did not include siblings and some parents expressed the view that they should have been included. The educational screening carried out six months after treatment indicated that two- thirds of the children who were screened needed some kind of extra support or adjustment at school. In addition, the educational follow up six months later showed that six of the seven children had a reading speed that was below average for their age. When it came to reading comprehension and basic arithmetic skills, most of the children had average results. Nevertheless, educational follow ups on all children are important after brain tumours, because there is a risk that reading and basic arithmetic skills will decline over time [22]. The neurocognitive tests that were carried out one year after the treatment ended revealed that more than half of the children whose cognitive levels were lower than average for their age had received multimodal treatment with surgery, radiation, and chemotherapy. The children who had just undergone surgery or chemotherapy showed an average general cognitive level, but significantly decreased information processing speed and working memory. Two-thirds of the children also needed extra support in school. Children who did not show any neurocognitive deficits one year after the end of treatment could be followed up using a brief screening battery, but the long-term follow up of all children who have been treated for brain tumours is warranted because cognitive problems often develop years after a brain tumour has been diagnosed and treated [23]. Although this study only focused on a small clinical group, the results support previous studies that have suggested a significant risk of neurocognitive sequelae in children treated for brain tumours [10]. We also identified that this risk is highest among children treated multimodally with surgery, radiation, and chemotherapy. However, there are also concerns about those children who just undergo surgery [11] or chemotherapy. When neurocognitive profiles provide evidence of specific problems with speed and working memory, these can mean that children are at risk of negative effects on learning and psychosocial development [24]. Such children are also more likely to need extra support in school than their classmates or their siblings [14,25] and to achieve lower marks than their classmates [26]. Although the aim of the study was not to examine the wellbeing or performance of the patients and their parents, the results of the neurocognitive and educational screening certainly support the need for close liaison between schools, hospitals and parents [27,28]. It is necessary for children who have had brain tumours to receive follow-up programmes that provide them with psychosocial support, cognitive development and academic achievement over a period of time. In order to accomplish and implement these programmes, the professional who participate, including physicians, psychologists, social workers and teachers, must have adequate competency as well as experience in their respective field. Furthermore, the results of our study support the need to develop a structured framework for performing prospective research on preventive and rehabilitation interventions. The strengths of this study were that it prospectively enrolled the children and it included all brain tumour types irrespective of malignancy, location and treatment. For example, tumours of a low malignancy grade that are located in the posterior fossa and are only treated with surgery can show late neurocognitive sequelae [27,30]. The study’s main weaknesses were the small number of patients, the short project period and that all the families did not complete the scheduled follow up.
Conclusions
The results of this project provide further indications of the great demand for a psychosocial, educational, and neurocognitive followup programme for children with brain tumours. The programme needs to be coordinated with the medical follow up and well anchored in the management of the healthcare organisations treating these children. In addition, involving competent, experienced and truly multi-professional teams may contribute to quality assurance in follow-up programmes for children with brain tumours and their families.
References
- Lannering B, Sandström PE, Holm S, Lundgren J, Pfeifer S, Samuelsson U, et al. Classification, incidence and survival analyses of children with CNS tumours diagnosed in Sweden 1984-2005. Acta Paediatr. 2009;98(10):1620-7.
- Limond JA, Bull KS, Calaminus G, Kennedy CR, Spoudeas HA, Chevignard MP, et al. Quality of survival assessment in European childhood brain tumour trials, for children aged 5 years and over. Eur J Paediatr Neurol. 2015; 19:202-10.
- Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003; 21:3255-61.
- Spoudeas HA, Charmandari E, Brook CG. Hypothalamo-pituitary-adrenal axis integrity after cranial irradiation for childhood posterior fossa tumours. Med Pediatr Oncol. 2003; 40:224-9.
- Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009; 101:946-58.
- Mavrides N, Pao M. Updates in paediatric psycho-oncology. Int Rev Psychiatry. 2014;26(1):63-73.
- James K, Keegan-Wells D, Hinds PS, Kelly KP, Bond D, Hall B, et al. The care of my child with cancer: parents' perceptions of caregiving demands. J Pediatr Oncol Nurs. 2002;19(6):218-28.
- Norberg AL, Steneby S. Experiences of parents of children surviving brain tumour: a happy ending and a rough beginning. Eur J Cancer Care (Engl). 2009;18(4):371-80.
- Ljungman L, Cernvall M, Gronqvist H, Ljotsson B, Ljungman G, von Essen L. Long-term positive and negative psychological late effects for parents of childhood cancer survivors: a systematic review. PLoS One. 2014; 9:e103340.
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399-408.
- Sønderkaer S, Schmiegelow M, Carstensen H, Nielsen LB, Müller J, Schmiegelow K. Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol. 2003;21(7):1347-51.
- Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. Mental retardation and developmental disabilities research reviews. 2006;12:184-91.
- Bruce BS, Chapman A, MacDonald A, Newcombe J. School experiences of families of children with brain tumors. J Pediatr Oncol Nurs. 2008;25(6):331-9.
- Mitby PA, Robison LL, Whitton JA, Zevon MA, Gibbs IC, Tersak JM, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003; 97:1115-26.
- Järpsten B, Taube K. DLS: Handledning för skolår 4-6. Stockholm, Sweden: Psykologiförlaget AB. 2010.
- Järpsten B. DLS: Handledning för skolår 7-9 och år 1 i gymnasiet. Stockholm, Sweden: Psykologiförlaget AB. 2005.
- Johansson M-G. LS Handledning Klassdiagnoser i läsning och skrivning för högstadiet och gymnasiet. Stockholm, Sweden: Psykologiförlaget AB. 2008.
- Adler B. Adler Färdighetstest i matematik: Version B-E. Malmö, Sweden: Kognitivt Centrum Sverige AB. 2008.
- Wechsler D. Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV). San Antonio, TX: The Psychological Corporation. 2003.
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III). San Antonio, TX: The Psychological Corporation. 2002.
- Gioia G, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventary of Executive Function, Psychological Assessment of Resources, Inc. 2000.
- Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256-63.
- Briere ME, Scott JG, McNall-Knapp RY, Adams RL. Cognitive outcome in pediatric brain tumor survivors: delayed attention deficit at long-term follow-up. Pediatric blood & cancer. 2008;50:337-40.
- Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30(1):65-78.
- Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Cancer. 2005; 104:1751-60.
- Lahteenmaki PM, Harila-Saari A, Pukkala EI, Kyyronen P, Salmi TT, Sankila R. Scholastic achievements of children with brain tumors at the end of comprehensive education: a nationwide, register-based study. Neurology. 2007;69:296-305.
- Anderson VA, Godber T, Smibert E, Weiskop S, Ekert H. Cognitive and academic outcome following cranial irradiation and chemotherapy in children: a longitudinal study. Br J Cancer. 2000;82(2):255-62.
- Bruce BS, Newcombe J, Chapman A. School liaison program for children with brain tumors. J Pediatr Oncol Nurs. 2012;29(1):45-54.
- Patel SK, Mullins WA, O'Neil SH, Wilson K. Neuropsychological differences between survivors of supratentorial and infratentorial brain tumours. J Intellect Disabil Res. 2011;55:30-40.
- Cantelmi D1, Schweizer TA, Cusimano MD. Role of the cerebellum in the neurocognitive sequelae of treatment of tumours of the posterior fossa: an update. Lancet Oncol. 2008;9(6):569-76.