Case Presentation
Rare Breast Myoepithelial Carcinoma with a Distinctive Genetic Profiling Study
Kerolos Abadeer1, Judith S. Kaur2, Mohd E.M. Ibrahim4 and Aziza Nassar3*
1Division of Nephrology and Hypertension, USA
2Division of Hematology and Oncology and Cancer Center and Breast Clinic, USA
3Department of Laboratory Medicine and Pathology, Mayo Clinic, USA
4Division of Pathology, Massey Cancer Center/VCU, USA
*Corresponding author: Aziza Nassar, Department of Laboratory Medicine and Pathology, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, FL 32224, USA
Published: 13 Mar, 2017
Cite this article as: Abadeer K, Kaur JS, Ibrahim MEM,
Nassar A. Rare Breast Myoepithelial
Carcinoma with a Distinctive Genetic
Profiling Study. Clin Oncol. 2017; 2:
1215.
Abstract
We describe a 74-year-old woman with no family history of breast cancer who presented with a high-grade myoepithelial carcinoma. Diagnosis was supported by immunohistochemical staining for both epithelial (ie, keratin) and myoepithelial (eg., p63, S-100, actin) differentiation. Because the patient reported that her family had an extensive history of neoplasm, a genetic study was ordered. To our knowledge, this is the first case to be reported in the literature with a complete genomic profiling study in which a 4-gene alteration has been detected. Molecular genetics profiling of the tumor using a genetic profile showed a genetic alteration in NF1 (L494fs*4), IRS2 amplification, RB1 loss, and TP53 (Y107D). The patient underwent lumpectomy with sentinel lymph node biopsy and chemotherapy treatment with carboplatin and taxol. She is alive and free of disease. Keywords: Breast; Genetics; Genomic profiling; Immunohistochemistry; Metaplastic carcinoma; Myoepithelial carcinoma; p63
Abbreviations
SMA; Smooth Muscle Actin
Introduction
Myoepithelial breast carcinoma is a rare breast cancer that arises from the myoepithelial
cells and shares overlapping phenotypic features with metaplastic carcinoma [1]. It has a unique
immunphenotypic profile with positive immunoexpression for cytokeratins (AE1/3), including
high-molecular weight keratin (CK 14, CK903) and myoepithelial differentiation (p63, S100), as
well as immunoreactivity to antibodies against myofilaments (Smooth Muscle Actin [SMA]). EGFR
expression is also common in myoepithelial carcinomas. They are typically negative for hormonal
receptors and HER2; therefore they are often considered triple negative breast cancer.
Myoepithelial carcinoma has a propensity for distant metastasis [1]. The molecular profiling of
these tumors has not been studied thus far. Herein, we describe a distinctive case of myoepithelial
breast carcinoma with its genomic profiling study showing alteration in 4 genes. No known
treatment regimen is available for this kind of tumor.
Case Presentation
A 74-year-old woman with hypertension presented to our medical center because of an
asymptomatic, non tender lump in her right breast and no palpable axillary or Supraclavicular
lymph nodes. The patient had been committed to having her annual mammogram, and her last
mammogram was less than a year ago. Nevertheless, neither she nor her doctors believed that
mammography screening helped to discover the current tumor. The patient had been pregnant
4 times and had given birth to 4 healthy children. She had an extensive history of cancer in her
family: Her father and 2 brothers had died of prostate cancer, and she had both a sister who had
lung cancer and a sister who had received a diagnosis of cancer of unknown type. No family history
of breast cancer existed, according to the patient. Her surgical history included a hysterectomy.
The remainder of the initial physical examination was unremarkable, and the patient had no other
concerns. She received a clinical diagnosis of carcinoma in the upper outer quadrant of the right
breast that was pathologically staged as a T2N0M0 tumor.
Three weeks after her original breast cancer diagnosis, the patient
underwent a right lumpectomy and right sentinel lymph node
biopsy. The excised sentinel node was found to be a single lymph
node measuring 1.2 cm in its greatest dimension. The gross breast
specimen was a red-yellow rubbery breast tissue fragment measuring
9.5 cm × 6.5 cm × 1.5 cm. The specimen was serially sectioned, showing cut
surfaces remarkable for an eccentrically located firm lesion measuring
2.4 cm × 1.5 cm × 1.8 cm. The remainder of the breast tissue was histologically
unremarkable.
Multiple paraffin-embedded sections were studied, which revealed
breast parenchyma infiltrated by high-grade malignant neoplasm,
composed of sheets and nests of mixed epithelioid and occasional
spindle cells with nuclei ranging from low grade to markedly
pleomorphic and of bizarre shapes (Figures 1 and 2). Several atypical
mitotic figures were found. Cystic spaces were noted that were filled
with basophilic and basement membrane–like materials (Figure 3).
Sections also showed a single benign lymph node with no evidence
of metastatic disease. Furthermore, sections of the surgical margins
surrounding the cancer had benign breast parenchyma with focal
atypical lobular hyperplasia despite no evidence of invasive disease.
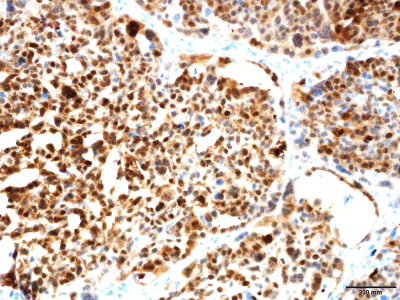
Immunohistochemistry results were strongly positive for
pancytokeratin (AE1/3) (Figure 4), SMA, smooth muscle myosin, p63
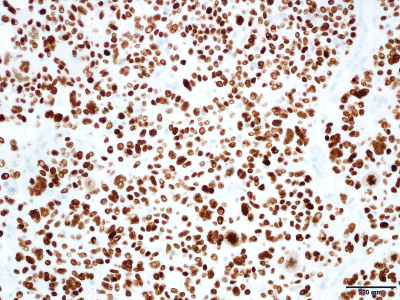
(Figure 5), and S-100 (Figure 6) while being negative for HER2 and
estrogen receptor and weakly positive for progesterone receptor. The final diagnosis was high-grade myoepithelial carcinoma of the breast.
Given the extensive family history of malignancy provided by
the patient, a thorough genetic study was ordered. The test was a
next-generation, sequencing-based assay that identifies genomic
alteration within hundreds of cancer-related genes (Foundation One;
Foundation Medicine). The current assay interrogates 315 genes, as
well as introns of 28 genes involved in rearrangement, both the genes
and introns known to be altered in cases with solid tumors. The test
result identified 4 genomic alterations, featuring NF1 (L494fs*4),
IRS2 amplification, RB1 loss, and TP53 (Y107D).
The genomic study also reported identification of certain variants
of unknown significance, including ASXL1 (A1312V) and CIC
(T295M). These variants may not have been adequately understood
or characterized in the literature, but we choose to include them in
our report in case they become clinically meaningful in the future for
targeted therapy.
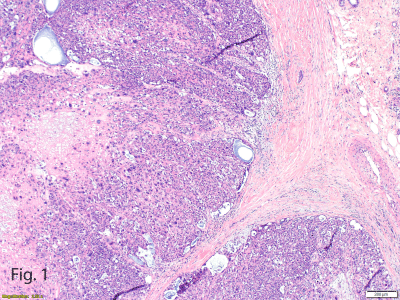
Figure 1
Figure 1
Pushing Borders of High-Grade Malignant Neoplasm at the
Interface With Normal Adjacent Breast Parenchyma Showing Microcystic
Spaces and Sheets of Epithelioid With Marked Cytologic and Nuclear Atypia
and Bizarre Nuclei (hematoxylin-eosin, original magnification ×10).
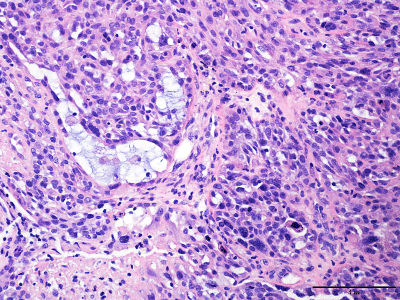
Figure 2
Figure 2
High-Grade Cytologic Atypia. Occasional sheets of spindle
neoplastic cells are admixed with epithelioid cells that exhibit marked highgrade
cytologic atypia with nuclear pleomorphism and bizarre nuclei and
show occasional mitotic figures (hematoxylin-eosin, original magnification
×40).
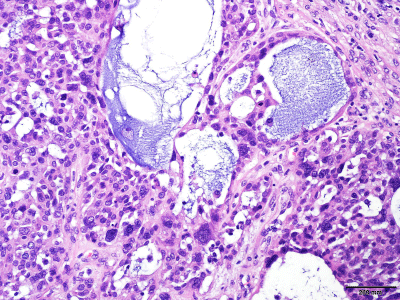
Figure 3
Figure 3
Basophilic and Basement Membrane–Like Material. Cystic spaces
with basophilic and basement membrane–like material (hematoxylin-eosin,
original magnification ×40).
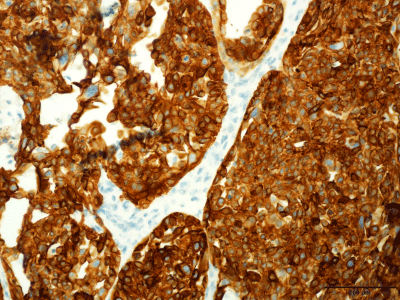
Figure 4
Figure 4
Pancytokeratin (AE1/3) Cytoplasmic Immunostain in the Neoplastic
Cells. Immunostain indicates epithelial differentiation (original magnification
×40).
Discussion
Myoepithelial carcinoma of the breast is a rare lesion, with about
38 cases reported in the English literature [2]. Therefore, the need
is becoming urgent to understand the nature of this rare entity. In
addition, our patient’s extensive family history of other cancers raises
questions about the genetic component of these types of lesions; only
a few studies in the literature have demonstrated the association
between EWSR1 gene rearrangements and myoepithelial carcinomas.
To our knowledge, this case report is the first of myoepithelial carcinoma of the breast that involved complete genomic evaluation
showing multiple genetic alterations, including NF1, IRS2
amplification, RB1 loss, and TP53. More molecular studies should be
conducted in this regard to illuminate the pathogenesis and genetic
profiling of this distinctive tumor.
Normal breast tissue architecture is composed of networks of
branching ducts lined with an inner luminal epithelial layer that
regulates the differentiation of the ductal epithelial cells from which
the more common adenocarcinoma of the breast originates. Yet, the
outer layer that envelops the branching ducts consists of myoepithelial
cells that rarely develop into the malignant lesion [3] discussed in the
present case report.
The structure of myoepithelial cells closely expresses their
function. Major components are desmosomes and hemidesmosomes,
by which the cells adhere to the luminal cells and the basement
membrane, respectively. They have a contractile function achieved by
the actin and myosin filaments embedded in their structure [1].
In 1991, Tavassoli [4] divided the myoepithelial lesions into 3
types: myoepitheliosis, adenomyoepithelioma, and myoepithelial
carcinoma. Adenomyoepithelioma was further categorized into
tubular, spindle-cell, and lobulated variants depending on the
architectural and histologic features. Only myoepithelial carcinoma
is composed of pure myoepithelial cells, and it is rare.
Even though the cytologic criteria to diagnose myoepithelial
carcinoma of the breast are still elusive, the World Health
Organization’s definition and certain descriptive histologic literature
describe the components of spindle and epithelioid cells as having
nuclear atypia and marked mitotic activity. In our present case, we
found epithelioid cells with bizarre nuclei. In addition, we recognized
cystic spaces filled with basophilic and basement membrane–like
materials, and the cytoplasm was pale eosinophilic. Other soft tissue
tumors that mimic myoepithelial carcinoma are leiomyosarcoma,
fibrosarcoma, and malignant phyllodes tumors. However, these
entities can be excluded by immunohistochemistry (eg., SMA,
desmin, CD10, CD34). Another important differential diagnosis is
metaplastic carcinoma of the breast, which is an uncommon breast
tumor but can be differentiated by immunohistochemical stains
(eg., AE1/3, MNF-116, p63) [1,5]. In addition, metaplastic breast
carcinoma has a unique genomic profiling. It is characterized by a
complex array of genomic alterations including multiple gene copy
number gains and losses, in addition to mutations in p53 (TP53)
(20%), loss of CDKN2A (p16) (20%) and PTEN (25%), as well as recurrent mutations of PIK3CA (40%), and of genes pertaining to the
Wnt pathway [6]. Furthermore, amplification and high polsomy of
the EGFR gene has been reported in 10% to 25% of metaplastic breast
cancers, as well as amplification of MYC (30%) [1,6].
Myoepithelial cell–derived neoplasms express both epithelial
and myoepithelial antigens on their cell surface. Therefore,
immunohistochemical antibodies can react to these antigen types and
especially to keratins and myofilaments. For example, antibodies to
basal keratins (5, 5/6, 14, and 17) react with most of the myoepithelial
tumors; in addition, SMA, muscle-specific actin, calponin, and S-100
are immunostains that can be used to detect the myoepithelial origin
of these tumors [2]. p63 is another important immunohistochemical
stain that can distinguish the myoepithelial cells of the breast,
but unlike other antibodies, p63 stains the nuclei rather than the
cytoplasm, as noted by the other antibodies [7].
In our case, the patient had extensive familial history of different
types of carcinomas—prostate (father and brothers), lung (sister), and
breast (index case). Four gene alterations have been reported in our
next gene sequencing study. Few studies have shown the involvement
of the EWSR1 gene rearrangement in many cases of myoepithelial
carcinoma, and in particular the cytologic appearances of epithelioid
cell nests are known to be associated with such specific gene fusions
as EWSR1-POU5F1 [8,9].
NF1 gene encodes a GTPase activating protein called
neurofiromin, which acts as a tumor suppressor gene by negatively
regulating the RAS signaling pathway [10,11]. Mutations in the NF1
gene cause the well-known autosomal dominant disorder called
neurofibromatosis type 1, which is associated with multiple types
of cancers, including sarcoma, glioma, hematologic neoplasm,
and breast carcinoma [12,13]. An association between NF1 gene
mutations and breast cancer is rare and has been reported in only
2% of breast tumors [14]. Nonetheless, in 1 study of 14 metastatic
breast cancers, the NF1 gene mutation was frequently reported [15].
Studies have shown that the risk of breast cancer is relatively high
for women having neurofibromatosis type 1 associated with germline
NF1 mutation [16,17].
The amplification of the IRS2 gene has been reported in cancer
cases and has been found in about 2% of invasive breast carcinoma
[18]. IRS2 encodes insulin receptor substrate 2, which is considered
a cytoplasmic signaling molecule. It also is essential in the negative
feedback loop between mTOR and Akt. Porter et al. [19] showed in
their study that high IRS2 expression was noted in invasive ductal carcinoma of the breast. Furthermore, increased IRS2 expression has
been associated with increased cell migration, leading to metastasis
and cancer spread in breast cancer cells [20]. This latter gene might
have contributed to the high propensity of metastasis associated with
these tumors.
Another altered gene found in our study was the RB1 gene. It
normally encodes the retinoblastoma protein that suppresses tumors
and acts as a negative regulator of the cell cycle [21]. RB1 gene
mutation or loss has been reported in approximately 2% of breast
cancer cases [18].
Because of the unique nature of myoepithelial carcinoma,
no therapy is known or approved by the US Food and Drug
Administration that is specific to the reported genomic alterations,
despite many studies having shown that myoepithelial carcinoma
has a high tendency for recurrence and distant metastasis. Therefore,
an effective treatment regimen is greatly needed. Most studies in the
literature have shown that constructive breast surgery combined with
adjuvant chemotherapy and radiotherapy may be effective [22].
Our genomic study reported 2 therapies with potential clinical
effect associated with NF1 gene mutation in another tumor type but
none in our patient’s tumor type. These 2 medications with potential
benefit are cobimetinib and trametinib, which are MEK inhibitors
approved by the US Food and Drug Administration for treatment
of unresectable or metastatic melanoma with BRAF V600E mutation
[23,24].
In conclusion, myoepithelial carcinoma of the breast is a rare
neoplasm that needs further molecular genetic study and review.
Unique cytologic morphologic characteristics could be detected
that mostly show nests of epithelioid cells with bizarre nuclei and
atypia. Immunohistochemistry can be conclusive in establishing the
diagnosis through both epithelial and myoepithelial antibodies (eg.,
keratin, p63, S-100). Myoepithelial carcinoma of the breast shows
association with some gene mutations and fusion, such as NF1 gene,
IRS1 amplification, RB1 loss, and TP53. No treatment regimens are
established in the literature regarding this rare entity.
Figure 5
Figure 5
P63 Nuclear Immunostaining in the Neoplastic Cells. Immunostain
confirms myoepithelial differentiation (original magnification ×40).
Figure 6
Figure 6
S100 Nuclear Staining in the Neoplastic Cells. Immunostain
confirms myoepithelial differentiation (original magnification ×40).
Acknowledgment
The authors are grateful for Foundation Medicine performing the genetic profiling of this distinctive tumor.
References
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2012.
- Liao KC, Lee WY, Chen MJ. Myoepithelial carcinoma: a rare neoplasm of the breast. Breast Care (Basel). 2010; 5: 246-249.
- Santhosh R, Padu K, Bipin Singh TH, Sharma MB, Singh THSC. Myoepithelial carcinoma of the breast. J Clin Diagn Res. 2013; 7: 1191-1193.
- Tavassoli FA. Myoepithelial lesions of the breast: myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991; 15: 554-568.
- Sauer T. Cytologic findings in malignant myoepithelioma: a case report and review of the literature. Cytojournal.2007; 4: 3.
- Ross JS, Badve S, Wang K, Sheehan CE, Boguniewicz AB, Otto GA, et al. Genomic profiling of advanced-stage, metaplastic breast carcinoma by next-generation sequencing reveals frequent, targetable genomic abnormalities and potential new treatment options. Arch Pathol Lab Med. 2015; 139: 642-649.
- Tse GM, Tan PH, Lui PC, Gilks CB, Poon CS, Ma TK, et al. The role of immunohistochemistry for smooth-muscle actin, p63, CD10 and cytokeratin 14 in the differential diagnosis of papillary lesions of the breast. J Clin Pathol. 2007; 60: 315-320.
- Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors: a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010; 49: 1114-1124.
- Jo VY. Myoepithelial tumors: an update. Surg Pathol Clin. 2015; 8: 445-466.
- Hattori S, Ohmi N, Maekawa M, Hoshino M, Kawakita M, Nakamura S. Antibody against neurofibromatosis type 1 gene product reacts with a triton-insoluble GTPase activating protein toward ras p21. Biochem Biophys Res Commun. 1991; 177: 83-89.
- Morcos P, Thapar N, Tusneem N, Stacey D, Tamanoi F. Identification of neurofibromin mutants that exhibit allele specificity or increased Ras affinity resulting in suppression of activated ras alleles. Mol Cell Biol. 1996; 16: 2496-2503.
- Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010; 12: 1-11.
- Patil S, Chamberlain RS. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist. 2012; 17: 101-116.
- Salemis NS, Nakos G, Sambaziotis D, Gourgiotis S. Breast cancer associated with type 1 neurofibromatosis. Breast Cancer. 2010; 17: 306-309.
- Craig DW, O’Shaughnessy JA, Kiefer JA, Aldrich J, Sinari S, Moses TM, et al. Genome and transcriptome sequencing in prospective metastatic triple-negative breast cancer uncovers therapeutic vulnerabilities. Mol Cancer Ther. 2013; 12: 104-116.
- Guran S, Safali M. A case of neurofibromatosis and breast cancer: loss of heterozygosity of NF1 in breast cancer. Cancer Genet Cytogenet. 2005; 156: 86-88.
- Wang X, Levin AM, Smolinski SE, Vigneau FD, Levin NK, Tainsky MA. Breast cancer and other neoplasms in women with neurofibromatosis type 1: a retrospective review of cases in the Detroit metropolitan area. Am J Med Genet A. 2012; 158A: 3061-3064.
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490: 61-70.
- Porter HA, Perry A, Kingsley C, Tran NL, Keegan AD. IRS1 is highly expressed in localized breast tumors and regulates the sensitivity of breast cancer cells to chemotherapy, while IRS2 is highly expressed in invasive breast tumors. Cancer Lett. 2013; 338: 239-248.
- Gibson SL, Ma Z, Shaw LM. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle. 2007; 6: 631-637.
- Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008; 8: 671-682.
- Endo Y, Sugiura H, Yamashita H, Takahashi S, Yoshimoto N, Iwasa M, et al. Myoepithelial carcinoma of the breast treated with surgery and chemotherapy. Case Rep Oncol Med. 2013; 2013: 164761.
- Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013; 123: 340-347.
- Jousma E, Rizvi TA, Wu J, Janhofer D, Dombi E, Dunn RS, et al. Preclinical assessments of the MEK inhibitor PD-0325901 in a mouse model of neurofibromatosis type 1. Pediatr Blood Cancer. 2015; 62: 1709-1716.