Case Report
Pancreas Divisum, Causing Recurrent Pancreatitis: A Case Report
Rizwan Sultan, and Nadeem Ahmed Siddiqui*
Department of Surgery, Aga Khan University Hospital Karachi, Pakistan
*Corresponding author: Nadeem Ahmed Siddiqui, Department of Surgery, Aga Khan University Hospital Karachi, Pakistan
Published: 31 Jan, 2017
Cite this article as: Sultan R, Siddiqui NA. Pancreas
Divisum, Causing Recurrent
Pancreatitis: A Case Report. Clin Oncol.
2017; 2: 1196.
Abstract
Pancreas divisum is one of the rare causes of recurrent pancreatitis. The diagnosis is a challenging
and diagnostic failure lead to recurrent episodes of pancreatitis and ultimately pancreatic failure.
Here the author is presenting a case of a young female with history of recurrent attacks of upper
abdominal pain which on work up diagnosed to have pancreas divisum. This patient was successfully
treated with ERCP. This report emphasizes the need of early suspicion of disease by clinician and
efficacy of minimally invasive procedures (ERCP) as definitive treatment option.
Keywords: Pancreas divisum; Recurrent pancreatitis; Endoscopic sphincterotomy
Abbreviations
CT: Computed Tomography; GI: Gastrointestinal; ERCP: Endoscopic Retrograde Cholangio Pancreatography; MRCP: Magnetic Resonance Cholangiopancreatography; PD: Pancreatic Divisum
Introduction
Pancreas divisum is one of the most commonly encountered congenital anomaly in hepatobiliary
system. The incidence is variable but it ranges from 5% to 14% of the general population [1].
The disease occurs s due to failure of fusion of the two embryonic parts of the pancreas i.e. non
fusion of dorsal and ventral ducts of pancreas resulting in openings at abnormal positions. This
abnormal opening of major pancreatic duct at minor papilla is not enough to completely drain the
duct secretions resulting in stasis of enzymes and premature activation of these enzymes results in
recurrent attacks of pancreatitis [2,3].
Most of the patients are asymptomatic and diagnosis is incidental. Abdominal ultrasound is
not a very useful modality for diagnosis. Computed Tomography (CT) of abdomen may help in
diagnosis but imaging of choice is cholangiography (ERCP or MRCP). MRCP being better as it is
noninvasive but ERCP has advantage in its diagnostic as well as therapeutic capacity. Treatment is
usually nonsurgical in the form of endoscopic sphincterotomy.
Here we are presenting a case of 24 year young female with history of recurrent attacks of
pancreatitis, which on workup is found to be secondary to pancreas divisum. She was successfully
treated with endoscopic sphincterotomy with pain free post procedure course. Report points out
the need of early disease suspicion and efficacy of endoscopic sphincterotomy for treatment of this
disease.
Case Presentation
Our patient was a 24 years old lady, presented in emergency department with complain of
sudden onset severe continuous epigastric pain for last four days. This pain was not radiating,
aggravated by food intake and relieved only with opioid analgesics. Pain was also associated with
non-bilious vomiting. She had episode of similar pain about 1 year back for which she was admitted
and managed as acute pancreatitis. She had history of acid peptic disease, anorexia, and malaise and
weight loss of 6 Kg in last 3 years. On examination she was dehydrated and jaundiced. Her pulse was
96/minute; BP was 117/68, febrile. Abdominal examination revealed tenderness in epigastria area
without any mass or palpable viscera. Rest of systemic examination was unremarkable.
Her initial laboratory workup showed TLC of 10600/mm3, total bilirubin 1.2 mg% (0.2-1.2)
with direct bilirubin 0.9 mg% (0.0 to o.4), alanine aminotransferase of 71 IU/l (normal 0-31 IU/l),
aspartate aminotransferase of 251 IU/l (normal 0-34 IU/l), alkaline phosphatase of 85 IU/l (12-38
IU/l), serum amylase was 99 IU/l (25-125 IU/l) and serum lipase was 167 IU/l (3-60 IU/l).
Ultrasonography (US) of abdomen surprisingly revealed a hypo
echoic solid mass in the head of pancreas resulting in atrophy of
pancreatic body and tail, dilatation of pancreatic duct and prominent
common bile duct suggestive of neoplastic process.
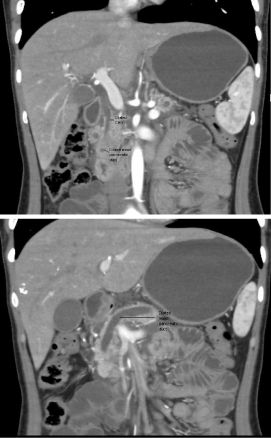
To further delineate this situation, CTscan of abdomen was done
which showeda hypo dense mass involving the uncinateprocess
of pancreas causing atrophy of body and tail of pancreas without
any infiltration into surrounding structures. Pancreatic duct and
common bile duct were prominent without dilatation of intrahepatic
biliary channels appeared dilated (Figure 1).
This CT scan was discussed in length with radiologists as it was
difficult to differentiate between pancreas Divisum and pancreatic
malignancy. Finally provisional diagnosis of pancreas Divisum was
made as there was a convincing evidence of opening of dilated major
pancreatic duct at minor papilla (Figure 2).
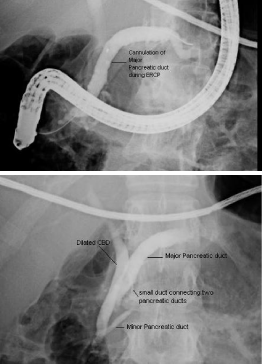
ERCP was done as diagnostic as well as therapeutic measure,
which confirmed the presence of minor papilla about 5 mm
proximal to the major papilla, major pancreatic duct was opening
at minor papilla while minor pancreatic duct was opening at major
papilla, Minor papillotomy was done and plastic stent placed in
major pancreatic duct. Patient tolerated the procedure well and no
immediate or delayed complication was identified.
Post procedure, patient had smooth recovery with significant
improvement in abdominal pain and appetite. She was discharged
on 3rd post procedure day. On clinic follow up of 6 months, she has
remained pain free and leading a normal healthy life.
Figure 1
Figure 1
CT scan showing hypodense mass involving the uncinateprocess
of pancreas and dilated common bile duct and pancreatic duct.
Discussion
Pancreatic Divisum is a congenital anatomical anomaly characterized by the lack of fusion of the ventral and dorsal parts
of the pancreas during the eighth week of fetal development. This
condition is found in 5% to 14% of the general population [1]. In large
retrospective study from India, pancreas divisum was more frequent
in patients with pancreatitis than in those with biliary diseases or
obscure abdominal pain (9 versus 2 percent).
The major pancreatic duct (Wirsung's duct), drains the secretions
from the head, body and tail of the exocrine pancreas, and ends at
the major duodenal papilla (hepatopancreatic ampulla); the accessory
pancreatic duct (Santorini’s duct) extends through the head of the
pancreas, crosses Wirsung's duct and ends at the minor duodenal
papilla; both pancreatic outlets are located on the medial wall of the
second part of the duodenum at a distance of approximately 10 to 15
mm from each other; the minor papilla are above, the major duodenal
papilla below.
In Pancreatic Divisum, the dorsal pancreatic section drains into
the minor duodenal papilla through the major pancreatic duct; the
ventral pancreatic duct, the smaller part of the pancreas, merges
with the common bile duct at the hepatopancreaticampulla. There
are two types of Pancreatic Divisum: complete (most common) and
incomplete (much less common), in which the ventral and dorsal
systems remain connected through small-caliber branch ducts.
Approximately 15 percent of cases of pancreas divisum are of the
incomplete type. However, the clinical implications of incomplete
pancreas divisum are the same as for classic (or complete) pancreas
divisum.
In PD, the increased incidence of acute and chronic pancreatitis
is caused by inadequate drainage of secretions produced by the body,
tail and part of the pancreatic head through an orifice which is too
small [4,5]. There is a group of patients with pancreas divisum who
are subject to recurrent bouts of seemingly idiopathic pancreatitis [6].
In these patients, the minor papilla orifice is so small that excessively
high intrapancreatic dorsal ductal pressure occurs during active
secretion, which may result in inadequate drainage, ductal distension,
pain, and, in some cases, pancreatitis. Although many times, patients
with pancreas Divisum remain clinically asymptomatic, but other
common forms of clinical presentations ranges from recurrent attacks
of varying degree of pancreatitis, bowel obstruction, ascites, jaundice,
shock and in its most severe form, can lead to shock. Alcohol seems
to be the triggering factor for attack of pancreatitis [6]. Our patient
presented with upper abdominal pain secondary to pancreatitis.
Diagnostic workup ranges for laboratory tests to imaging
modalities. Laboratory work up may show raised amylase or lipase
levels indicating episode of pancreatitis, but they are not specific for
diagnosing pancreas Divisum. Imaging modalities which may help
in diagnosis include ultrasound, CT scan abdomen but definitive
diagnosis is made with some form of cholangiography either ERCP
or MRCP. PD is most of the times diagnosed from MRCP but
important point to note here is that currently used 64 slicer CT scan
is a good modality to diagnose PD like in our case, especially when the
diagnosis is not being suspected [7].
The usual therapeutic solution for symptomatic Pancreatic
Divisum is a sphincterotomy of the minor duodenal papilla, which
decongests Wirsung's duct [8,9]. Clinical improvement with such
treatment has been observed in up to 75 percent of patients. Rarely,
in selected cases only, is surgical treatment indicated: surgical
sphincterotomy, draining or even partial pancreatectomies and their
results are comparable with those achieved by endoscopic procedure
[6].
Figure 2
Figure 2
ERCP showing presence of minor papilla about 5 mm proximal to
the major papilla, major pancreatic duct was opening at minor papilla (upper
figure) while minor pancreatic duct was opening at major papilla (lower
figure).
References
- Liao Z, Gao R, Wang W. A systematic review on endoscopic detection rate, endotherapy, and surgery for pancreas divisum. Endoscopy. 2009; 41: 439-444.
- Vasile D, Grigoriu M, Turcu F. Pancreas divisumea rare cause of chronic pancreatitis. Chirurgia Bucur. 2007; 102: 83-87.
- Gonoi W, Akai H, Hagiwara K. Pancreas divisum as a predisposing factor for chronic and recurrent idiopathic pancreatitis: initial in vivo survey. 2011.
- Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A. Clinical implications of incomplete pancreas divisum. JOP. 2006; 7: 625-630.
- Ng WK, Tarabain O. Pancreas divisum: a cause of idiopathic acute pancreatitis. CMAJ. 2009; 180: 949-951.
- Gologan E, Achitei D, Bocan R, Balan G. Pancreas divisum pancreatitis: a case report. Abdom Imaging. 2011; 36: 215–217.
- Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007; 129-133.
- GA L. Acute recurrent pancreatitis. Can J Gastroenterol. 2003; 381-383.
- Kamisawa T. Clinical significance of the minor duodenal papilla and accessory pancreatic duct. J Gastroenterol. 2004; 39: 605–615.