Case Report
Two Solid Tumours in a Transplanted Kidney
Katarzyna Sklinda1*, Joanna Ludwikowska1, Michał Frączek1, Jarosław Ratajski2, Bartosz Mruk1 and Jerzy Walecki1,3
1Department of Radiology, Szpital Kliniczny MSWiA, Poland
2Department of Urology, Szpitala Bielańskeigo im. ks. J. Popiełuszki w Warszawie, Poland
3Department of Radiology, Samodzielny Publiczny Szpital Kliniczny, Poland
*Corresponding author: Katarzyna Sklinda, Department of Radiology, Szpital Kliniczny MSWiA, Poland
Published: 31 Jan, 2017
Cite this article as: Sklinda K, Ludwikowska J, Frączek M,
Ratajski J, Mruk B, Walecki J. Two Solid
Tumours in a Transplanted Kidney. Clin
Oncol. 2017; 2: 1194.
Abstract
A 35-year female otherwise healthy patient 2 years after renal transplantation underwent routine
ultrasound examination which revealed two solid lesions within transplanted kidney. The
transplantation was performed due to end-stage renal disease caused by chronic glomerulonephritis.
The patient received graft from unknown cadaveric donor, the surgery was uneventful. After
transplantation the patient received maintenance immunosuppression in form of combination of
tacrolimus and mycophenolate mofetil. The patient was referred to MR imaging which confirmed
presence of two solid lesions in transplanted kidney showing different radiologic features. The
performed biopsy confirmed malignant character of both of them - first lesion was lymphoma and
second RCC which was removed through NSS. No other lesions were revealed. The allograft was not
removed. After surgery the patient received further treatment.
Keywords: Kidney; Renal transplantation; Neoplasm; Tumour; RCC; Lymphoma; PTLD
Introduction
Transplant recipients have higher prevalence of various malignancies which is considered to be
correlated with four [1] potential situations. Influence of chronic use of immunosuppressive agents
on neoplastic process is known from 1970s [1]. Transplant patients can develop malignancy de novo
or it may be transmitted to the recipient with donor organ. Some patients are previously diagnosed
with cancer. All this problems present clinical challenge including diagnosis and management of
malignancies.
One of the most common malignancy which occurs in 1% of renal allograft recipients is Post-
Transplant Lymphoproliferative Disorder (PTLD).
Risk factors of PTLD are associated with Epstein-Barr viral infection, concomitant
cytomegalovirus infection and immunosuppressive regime. For instance allograft involvement is
more common with azathioprine therapy [1].
PTLD range from low-grade, benign lymphoid hyperplasia to high grade malignant non-
Hodgkin's lymphoma (NHL).Prognosis depends on the grade of the lymphoma, and cell
type. Approximate five-year survival is ~35% [1].
There is a tendency of development aggressive non – Hodgkin lymphoma. Large analysis by
Opelz and Dohles confirmed that recipients of solid organs transplants are in higher risk than
general population (20-fold increase for kidneys) and the risk of PTLD is highest in the first year
following operations [1].
Image of renal allograft involvement is similar to the one seen in malignant lymphoma in
general. The kidney involvement is typically seen as a part of multiorgan process but it also may be
seen as a primary disease [1].
MRI is the optimal method of imaging for patients with renal insufficiency or iodinated
contrast allergy. Lymphoma especially occurs near hilum [1]. Typical features in MR include mass/masses which are hypointense to renal parenchyma on T1-weighted images, iso- or hyperintense
to renal parenchyma on T2-weighted images and show poor enhancement or sometimes delayed
enhancement after intravenous gadolinium administration comparing to renal parenchyma [2].
Next most common malignancy are renal and transitional cell (urothelial) carcinoma, which
have both higher prevalence in transplant recipients and can occur in allograft or native kidneys. The pathogenesis of renal cell cancer is uncertain. It can be correlated with
polycystic kidney disease or acquired cystic kidney disease. Generally
RCC is in the transplant population is 10 - fold more common [2].
Histopathologic examination confirmed diagnosis of RCC and
lymphoma in the same transplanted organ.
Coincidence of two different tumours in transplanted kidney, as
in this case, is extremely rare.
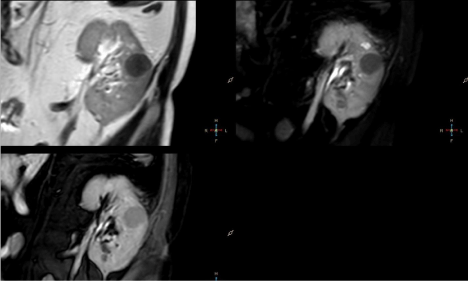
Figure 1
Figure 2
Figure 2
DIXON fat-only sequence revealed no fat content and DIXON
water-only sequence showed water content of normal renal parenchyma.
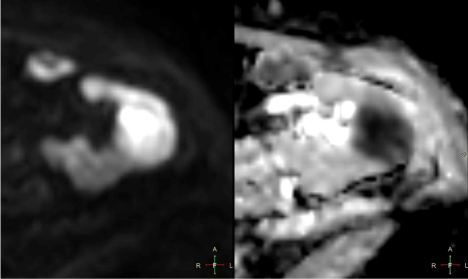
Figure 3
Description of MRI Examination Protocol
MRI examination was performed on 3T Philips Ingenia scanner.
Protocol included T2W TSE, DIXON, BTFE SPIR, T2 SPAIR, DWI sequences before IV contrast administration and multiphase
e-THRIVE, DIXONsequences after IV administration of 20ml
Multihance (Gadolinium-based contrast agent). In order to decrease
bowel movement artefacts, prior to examination 20 mg of Buscolysin
was injected intravenously.
Examination revealed two solid parenchymal lesions in
transplanted kidney with different characteristics.
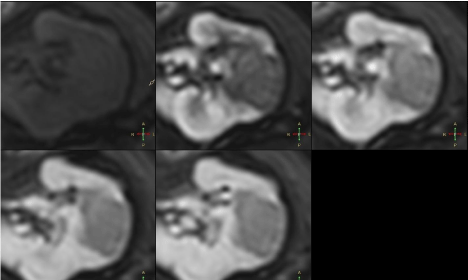
Figure 4
Figure 4
After contrast administration lesion showed subtle contrast
enhancement, hypointense rim compared to renal parenchyma.
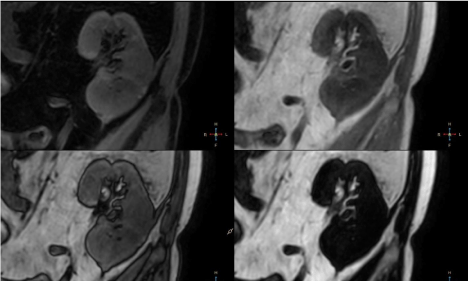
Figure 5
Figure 6
Figure 6
Lesion appeared isointense on DIXON in-phase sequence with
irregular areas of increased signal intensity and hypointense peripheral rim.
MRI Findings
First lesion, round in shape with sharp margins, 27mm in
diameter, was located in the medial part of the kidney and showed
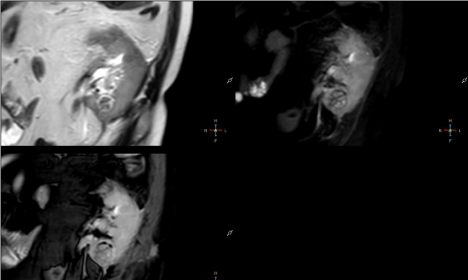
homogeneously decreased signal intensity on T2W, T2W SPAIR and BTFE SPIR images compared to renal parenchyma (Figure 1).
DIXON in-phase images showed signal intensity of normal
kidney parenchyma with no signal drop in out-of-phase images.
DIXON fat-only sequence revealed no fat content and DIXON wateronly
sequence showed water content of normal renal parenchyma
(Figure 2).
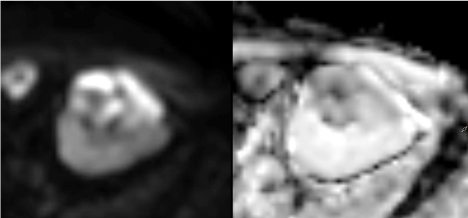
Lesion exhibited high signal on DWI b = 800 and markedly
restricted diffusion on ADC maps (Figure 3). After contrast
administration lesion showed subtle contrast enhancement,
hypointense rim compared to renal parenchyma (Figure 4).
Second, ball-shaped lesion, 20 mm in diameter, was located in
the lower pole of the kidney and showed inhomogenously decreased
signal intensity on T2W, T2W SPAIR and BTFE SPIR images with
peripheral rim hypointense on T2 TSE and hyperintense on T2
SPAIR and BTFE SPIR images. T2W SPAIR sequence showed
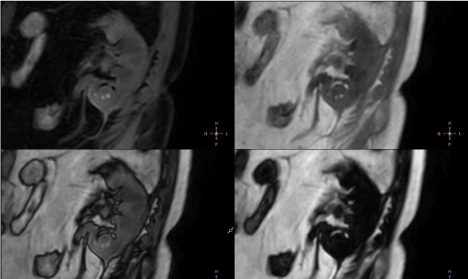
irregular hypointense septa within lesion (Figure 5). Lesion appeared
isointense on DIXON in-phase sequence with irregular areas of
increased signal intensity and hypointense peripheral rim (Figure 6).
DIXON water-only sequence revealed small fluid areas in the lower
aspect of the lesion and crescent shape fat signal in the upper aspect
of the lesion. Signal drop in out-of-phase images surrounding fat
content was noted (Figure 6).
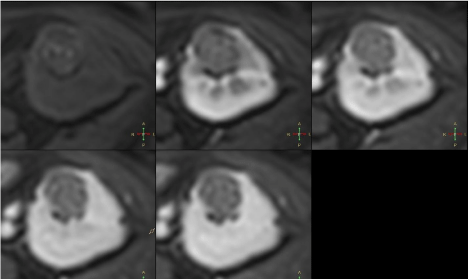
Lesion exhibited regions of high and low signal on DWI
corresponding to inhomogenously restricted diffusion on ADC
maps (Figure 7). After administration of contrast agent no contrast
enhancement was noticed (Figure 8).
Figure 7
Figure 7
Lesion exhibited regions of high and low signal on DWI
corresponding to inhomogenously restricted diffusion on ADC maps.
Differential Diagnosis
Lesion in the medial part of kidney
Renal Lymphoma: Most common malignant tumour in
transplanted kidney. Usually presents as multiple homogenous
implants although can also appear as solitary mass.
Typically presents as homogenous mass, iso- to hypointense on
T1W images and hypointense on T2W images. Typically strongly
restricts diffusion due to densely packed cells. Shows mild contrast
enhancement.
Lesion in the lower pole
Although differential diagnosis of solid kidney lesions is broad,
one should always consider malignant process in immunosuppressed
patient.
Radiological appearance of renal cell carcinoma varies greatly.
Typically it has isointense to low signal on T1W, with parts of increased
signal intensity due to internal haemorrhage (methemoglobin signal).
Papillary RCC has decreased signal T2 intensity opposed to Clear Cell
RCC with typically increased T2 signal. Papillary RCC often appears
with pseudocapsule presenting as hypointense rim on T1W and T2W
images. Some RCC can mimic AML due minimal fat content and
present loss of signal on out of phase images. RCC presents partly
with restricted and partly facilitated diffusion. Contrast enhancement
usually reveals inhomogenously enhancing lesion, usually relatively
hypovascular compared to normal renal parenchyma. Radiological
picture of renal cell carcinoma in immunosuppressed patient can be
different from that of individual with normally functioning immune
system.
Renal oncocytoma is often very difficult to distinguish from RCC.
It is encapsulated tumour typically isointense to low signal intensity
on T1W and intermediate signal intensity on T2W images. On T2W
images central scar area can appear hyperintense and mimic central
necrosis. True necrotic, hemorrhagic, calcific or cystic component is
very rarely seen in oncocytoma. DWI and contrast enhancement also
shows similar pattern to RCC.
Figure 8
Renal Lymphoma
Described above
Angiomyolipoma: AML can appear similar to RCC with minimal
fat content. AML shows high, heterogeneous signal intensity on T1
and T2W images, signal loss in out of phase images. It can show
signs of internal haemorrhage but very rarely presents with internal
septa. Diffusion weighted imaging and contrast enhancement cannot
reliably differentiate AML from malignant and other benign tumours.
Radiologic diagnosis
Lesion located in the medial part of the kidney is solid, shows
strong diffusion restriction and little contrast enhancement.
Considering morphology and clinical history of the patient renal
lymphoma must be suspected.
Lesion located in the lower pole of the kidney is mainly solid with
some septa, small cystic/water component and minimal fat content.
Shows no contrast enhancement. Considering morphologic features
and immunosuppression of the patient, radiologic diagnosis of RCC
was suggested.
Conclusion
Although occurrence of two different tumours in one organ is rare it must always be taken into consideration especially in immunosupreessed patient.
Images Description
1 – Coronal T2 TSE, T2 SPAIR and BTFE SPIR images. Roundshaped
lesion located in medial part of kidney appears homogenously
hypointense compared to renal parenchyma.
2 – Coronal DIXON water-only, in-phase, opposed-phase and fat
only images. Lesion appears isointense to normal renal parenchyma
with no fat content and no signal drop-out in opposed phase.
3 – Axial DWI b800 sequence and ADC maps. Lesion shows
high signal intensity on DWI b800 corresponding to strong diffusion
restriction confirmed on ADC maps.
4 – Axial multiphase e-Thrive sequences. Lesion in the medial
portion of the kidney shows no contrast enhancement in any phase.
5 – Coronal T2 TSE, T2 SPAIR and BTFE SPIR images. Roundshaped
lesion located in the lower pole of the kidney appears
inhomogenously hypointense compared to renal parenchyma. Note
peripheral rim around the lesion – hypointense on T2 TSE and
hyperintense on T2 SPAIR and BTFE SPIR images and hypointense
septa best appreciated on T2 SPAIR images.
6 – Coronal DIXON water-only, in-phase, opposed-phase and fat
only images. Lesion appears isointense on DIXON in-phase sequence
with irregular areas of increased signal intensity and hypointense
peripheral rim. DIXON water only sequence reveals small liquid
areas in the lower aspect of the lesion and crescent shape fat signal
in the upper aspect of the lesion - note signal drop in opposed-phase
images.
7 - Axial DWI b800 sequences and ADC maps. Lesion shows
areas of high and low signal on DWI b800 sequence corresponding to
inhomogenous diffusion restriction on ADC maps.
8- Axial multiphase e-Thrive sequences. Lesion in the lower pole
of the kidney shows no contrast enhancement in any phase.
References
- Stallone G, Infante B, Grandaliano G. Management and prevention of post-transplant malignancies in kidney transplant recipients. Clinical Kidney Journal. 2015; 8: 637-644.
- Penn I. Malignancies associated with renal transplantation Second malignant neoplasms associated with immunosuppressive medications. Urology. 1977; 10: 57–63.
- Brennan KC, Lowe LH, Yeaney GA. Pediatric central nervous system post transplant lymphoproliferative disorder. AJNR Am J Neuroradiol. 2005; 26: 1695-1697.
- Halkos ME, Miller JI, Mann KP. Thoracic presentations of post transplant lymphoproliferative disorders. Chest. 2004; 126: 2013-2020.
- OpelzG, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant.2004; 4: 222–230.
- Tsang K, Kneafsey P, Gill MJ. Primary lymphoma of the kidney in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1993; 117: 541-543.
- ChangSS, Nayak R, Cookson MS. Lymphoma presenting as a solitary renal hilar mass. Urology. 2002; 59: 134–135.
- Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: patterns of disease with pathologic correlation. Radiographics. 2006; 26: 1151-1168.
- Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004.