Review Article
Advances in the Management of Soft Tissue Sarcomas - Focus on Emerging Therapies
Richard Maguire1*, Vladimir Andelkovic2,3, Hanumant Chouhan1 and Gregory J Nolan1
1Department of Surgery, Gold Coast University Hospital, Australia
2Department of Medical Oncology, Princess Alexandra Hospital, Australia
3Department of Medicine, University of Queensland, Australia
*Corresponding author: Richard Maguire, Department of Surgery, Gold Coast University Hospital, Australia
Published: 19 Jan, 2017
Cite this article as: Maguire R, Andelkovic V, Chouhan
H, Nolan GJ. Advances in the
Management of Soft Tissue Sarcomas
- Focus on Emerging Therapies. Clin
Oncol. 2017; 2: 1189.
Abstract
Soft Tissue Sarcoma (STS) encompasses a number of different clinical entities and is a rare form of cancer. This review focuses on treatment of extremity and trunk STS (excluding gastrointestinal stromal tumors), focusing on more recent treatment updates. Early disease is managed by surgical resection as a definitive curative procedure, sometimes in combination with radiotherapy. Newer techniques have decreased the burden of resection and allowed safer delivery of radiation. Adjuvant chemotherapy cannot be recommended as a general approach but is used in individual cases with high-risk, chemosensitive tumours. Advanced disease poses even more dilemmas in terms of management, and outcomes are generally poor. Research in systemic treatment has not had major breakthroughs in field of STS in the last 30 years. Doxorubicin is the standard first line treatment, while ifosfamide and dacarbazine are the most often used second line therapy. Eribulin and Trabectedin have been approved as second line therapies in more recent times. Targeted therapy is of particular interest and newer agents are becoming available for use. Pazopanib is used in nonadipocytic sarcoma after progression on chemotherapy. A number of newer agents are harbouring interest including olarutamab, cediranib and aldoxorubicin. The major focus in oncology research is currently in the field of immunotherapy, and more mature data in STS is awaited. Ultimately management is best performed in a specialist unit with a multi-disciplinary approach to improve patient outcomes. Clinical trial participation remains the preferred approach for patients with advanced disease.
Introduction
STS encompasses a number of different subtypes with up to 50 different forms based upon the related cell type [1]. These tumours originate from pluripotent cells of the mesenchyme and are extremely rare making up less than 1% of overall cancer diagnoses [1,2]. Surgical resection with adequate margins remains the key to treatment of localised disease, however advanced disease requires a multimodal approach [3]. Chemotherapy regimens over time have become more suited to treatment of the disease particularly with the addition of targeted therapy. Such newer agents are aimed at the different subsets of soft tissue sarcoma and have shown promising results in certain groups [1]. Radiotherapy as an adjunct to surgery is commonly used particularly in tumours of the extremity, but adjuvant chemotherapy has not been shown to increase survival [4]. This paper will aim to explore current treatment options, with particular attention to the emerging therapies now available.
Presentation and Work-up
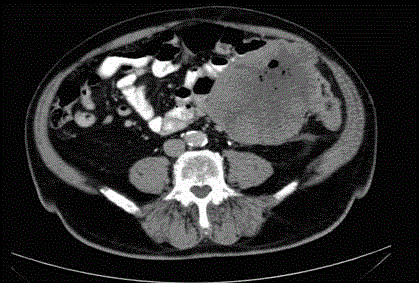
STS can present in a multitude of different ways, depending upon location and size. The usual presenting symptom, is a mass, generally not painful, which has increased in size over time. If the tumour grows into surrounding structures, then this may cause symptoms including altered sensation, lymphatic obstruction or bowel and/or urinary symptoms [1,5]. Once patients have been seen by a specialist, the next step in further assessment will be imaging to define the mass. Computed Tomography (CT) will provide information related to likely source and relation to other structures (Figure 1). To stage the patient the chest, abdomen and pelvis should be imaged with CT as well [1,5]. CT angiogram could be useful, if there are adjacent vascular structures at risk, particularly if resection is planned. Magnetic Resonance Imaging (MRI) can assist in differentiating the tissue of origin and has been found to be particularly useful when assessing soft tissue sarcoma of the extremities. PET CT is not always a part of the routine work-up, though it can show malignant spread or provide other differentials for the mass [1,5]. Imaging techniques will usually give sufficient information, but if management will be affected then biopsy can be done to confirm diagnosis. In tumours of the periphery this can usually be done by core biopsy of the tumour, which will generally give an adequate sample. Fine Needle Aspirate (FNA) will often not provide sufficient tissue for tumour assessment and is not recommended. The tract for biopsy should be made in a way that it can be excised once definitive resection is planned. In soft tissue sarcoma of the retroperitoneum, biopsy should be undertaken with care, due to risk of tumour seeding to surrounding structures [1,5]. A recent retrospective analysis however showed no significant risk of tumour seeding if retroperitoneal sarcomas were biopsied [6].
Figure 1
Figure 1
Axial CT image – showing large retroperitoneal sarcoma invading
into small bowel mesentery.
Distribution and Staging
Given the origin of soft tissue sarcoma, many regions and tissue
types can be affected by tumour. Most lesions affected either the
upper or lower limb, which can account for up to 60% of tumours.
The retroperitoneum or peritoneal space are the next most common
location for these cancers. Head and neck lesions, plus those of the
trunk are less common than the above, but are still seen in up to 10%
of presentations [1,5].
As previously mentioned over 50 subtypes exist, though some
are encountered more frequently. Liposarcoma and Leiomyosarcoma
are the most common types of soft tissue sarcoma. Accounting
for approximately 20% of tumours respectively. Fibro sarcomas
and synovial sarcomas are the next most common lesions that are
encountered in clinical practice. Gastro-intestinal stromal tumour
was once considered a form of Leiomyosarcoma, but they are now
considered separately to soft tissue sarcomas entirely [5].
Staging of soft tissue sarcoma gives prognostic information and
is adapted from the IUCC -AJCC TNM system (International union
against cancer; American joint committee on cancer). Tumour grade
is divided into 4 categories (I-IV) and further separated into low (I,II)
and high grade (III,IV). This is assessed on histological review of
features such as mitotic grade, necrosis, presence of de-differentiation,
cellular changes and content of stroma. Low grade lesions have a
significantly lower rate of distant metastasis (<15%) compared to
high grade tumours (>50%). TNM classification of sarcoma assesses
the size of lesions and depth of invasion for T staging, presence of
nodal disease or distant metastasis completes staging [1,5] (Table 1
and 2).
Surgery
Given the distribution of STS, surgical resection with clear margins remains challenging even in the hands of the most
experienced surgeon. Tumours of the retroperitoneum constitute
15% of all soft tissue sarcoma. Wide local excision is difficult due
to the need to preserve vital structures in the vicinity [4]. In the
management of low-grade tumours, resection with wide margins is
the standard of care. A recent Australian study showed close margins
of >1mm did not affect outcomes, though this is not widely accepted
[3]. Wider margins of at least 2-3cm have been shown in numerous
papers to reduce rates of local recurrence. It is therefore suggested
that specialist surgical teams be involved in resection with general
surgical and orthopaedic sarcoma experts [3,4]. Soft tissue sarcoma
disseminates via haemotogenous spread, therefore extensive lymph
node dissection is not required [5].
STS form pseudo capsule from the tissue of origin, which has
been compressed by growth. Malignant cells can spread beyond
this, meaning a macroscopically clear margin does not always mean
complete excision [4]. Wide local excision does not simply involve
the removal of the visible tumour but includes surrounding tissue to
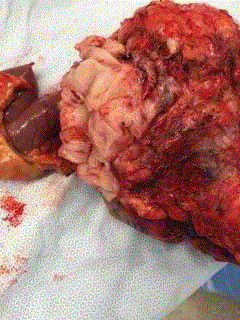
reduce recurrence [3], (Figure 2) Patient outcomes have been shown
to be improved when sarcoma is managed in specialised units with a
multidisciplinary approach [7].
Dickinson et al. [3], suggested that in low-grade sarcomas their
specialist unit did not routinely use adjuvant chemoradiotherapy
and this did not affect outcomes. It was also suggested that to avoid
morbidity in these patients undertaking radical resection, that vital
structures do not need to be resected just to obtain wide margins
[3]. The risk of local recurrence vs. overall quality of life need to be
weighed up in deciding what is best for the patient. Clark et al. [4]
suggested when resection is combined with radiotherapy in sarcoma
of the extremity, a recurrence rate as low as 4% is possible, if margins
are left positive to allow the limb to be preserved. In management of tumours of the head and neck there are many challenges related
to obtaining clear margins and acceptable cosmesis [8]. MOHS
micrographic surgery has been used in this setting. This involves
taking small segments of tissue at the resection margins, sending them
for frozen section and having them reviewed by a histopathologist
intraoperatively [8]. The aim is to confirm clear microscopic margins
in real time and to limit the amount of normal tissue taken with
the tumour. Loghdey et al. [8] showed a significant reduction in
local recurrence in patients who underwent MOHS surgery for
Dermatofibrosarcoma Protuberans (1.1%) versus traditional wide
local excision (7.3%). Plastic and reconstructive surgeons are best
involved in tumours of the head and neck to utilise such techniques
[9]. Tissue expanders can be used to fill such defects from the tumour
resection and to improve aesthetics [10].
Figure 2
Figure 2
Operative Specimen showing large poorly differentiated sarcoma,
resected en bloc with surrounding small and large bowel.
Table 1
Radiotherapy
Radiotherapy can be used in limb-sparing surgery to reduce local recurrence rates. It is most often an adjunct to surgical resection in high grade sarcoma [3]. This can be given as neoadjuvant or more commonly adjuvant treatment and is particularly useful if margins are suspected to be close or involved [4]. Mann et al. [11] suggested however that morbidity is worse when radiotherapy is given preoperatively and there are increased rates of secondary procedures [5]. Site of the tumour certainly affects its safe use, particularly in relation to retroperitoneal sarcoma, where safe delivery of radiation is problematic [3]. When circumstances do not permit surgical excision of tumour, radiotherapy has been used as a primary treatment for sarcoma. Higher fraction external beam radiation is required to be effective though in this setting, and management is aimed to prevent further local spread of disease [5]. Delivery methods include external beam radiation and brachytherapy, dependent upon site and accessibility of the tumour. There is limited evidence to suggest which provides better results, therefore specialist decision will guide the choice of delivery method [3]. Low grade tumours often will not require radiotherapy treatment if clear margins have been obtained.4 Though some studies have examined its use in such settings, evidence remains in favour of its use with high grade or large tumours [5]. Retroperitoneal soft tissue sarcomas pose resection dilemmas as well as challenges in the delivery of radiotherapy. Major vessels of the alimentary tract, bowel and solid organs are at risk of collateral damage and should be spared where possible [3]. To assist with the administration of radiotherapy a novel technique of leaving a tissue expander within the tumour bed after resection has been proposed. This allows the field for external beam radiation to remain clear of structures such as bowel, increasing the overall fraction that can be delivered safely [9]. High local recurrence rates in retroperitoneal sarcomas lead to the development of this technique. Data to prove this techniques overall efficacy is not particularly strong, though it has been shown to be safe. A recent observational study suggested higher dose radiation was able to be given in patients who had tissue expanders inserted and also suggests a significant reduction in local disease recurrence rates [10]. From this it can be said that radiotherapy is best utilised in the adjuvant setting particularly in tumours of the extremity. Low grade lesions should not need radiotherapy, when wider excision is possible, except in larger lesions greater than 5cm [5]. Lindberg et al. [11], though showed that in limb preservation surgery adjuvant radiotherapy provided recurrence rates as low as 6% in low grade lesions, that had radiotherapy post-operatively. Poorly differentiated tumours as expected have higher recurrence rates, though adjuvant radiotherapy has been suggested to be effective in such lesions [4,11].
Table 2
Table 3
Systemic Treatment
Adjuvant treatment: Adjuvant chemotherapy in soft tissue
sarcoma remains a controversial topic. Early trials utilizing
doxorubicin have suggested benefit in using adjuvant chemotherapy
but none of the trials showed statistically significant difference
in overall survival. Meta-analysis of these early trials, conducted
by SMAC and published in Lancet in 1997 showed statistically
significant difference in relapse free survival and recurrence rates,
but no statistically significant difference in overall survival [2].
Ifosfamide, although known to be effective agent in STS, was not
used in the early adjuvant trials because of dose limiting toxicity,
leading to acute haemorrhagic cystitis. In the 80’s, after Mesna
was shown to be effective in preventing haemorrhagic cystitis,
subsequent trials incorporated ifosfamide. The updated analysis that included newer generation trials with ifosfamide showed survival
benefit for combination protocols that included ifosfamide [12].
However, two large EORTC trials, both using doxorubicin and
ifosfamide based protocols did not show difference in survival with
adjuvant chemotherapy [13-15]. Based on these results, adjuvant
chemotherapy is not recommended as standard approach, but it can
be considered on the individual basis, depending of the risk features
and chemosensitivity of tumor.
Advanced disease: Metastatic soft tissue sarcoma is a terminal
diagnosis for most of the patients apart for the select few that are
amenable to metastasectomy. The treatment intent and choice
of treatment modality is complex and requires multidisciplinary
involvement that includes medical and radiation oncology as well
as palliative care and ssurgical teams. Reported median survival for
advanced STS is in the range of 12-18 months, although it varies
significantly, depending on the subtype of tumour, disease burden
and performance status [16]. A recently published Australian
multicenter study reported that one-third of patients with advanced
disease received systemic treatment, and a similar percentage of
patients underwent radiotherapy or metastasectomy for palliation
[17]. The choice of treatment modalities will depend on the site and
extent of disease, presence of symptoms and performance status,
and predicted chemosensitivity and Radiosensitivity of the tumour.
Chemosensitivity varies depending on the subtype. Liposarcoma
and synovial sarcoma are considered more chemosensitive whereas
undifferentiated pleomorphic sarcoma does not respond well to
chemotherapy [16,18], (Table 3).
Table 4
Table 5
Table 6
Chemotherapy
For the patients deemed fit for systemic treatment, standard of care is cytotoxic chemotherapy for the majority of tumour subtypes.
Doxorubicin is the first line treatment of choice in advanced soft tissue
sarcoma [1]. It was one of the first agents to show activity in sarcoma
and the the response rates average in the range of 15-20%, depending
on the study [19,20]. Increased response rates have been achieved
with adding another cytotoxic agent (ifosfamide, [19], dacarbazine
[20]) to doxorubicin. No trial has shown improved survival advantage
and toxicity is increased with combination treatment. Based on these
results, combination chemotherapy remains a reasonable choice
in fit patients with significant burden of symptoms, where tumour
shrinkage will likely result in improved quality of life; but can not be
recommended as general principle.
Other anthracyclines that were investigated for STS include
pegylated liposomal doxorubicin (PLD), and aldoxorubicin. A
phase II randomized study that compared PLD with standard
doxorubicin found a similar response rate between groups (10 and
9 percent resepectively). There was also a different adverse event
profile – haematological toxicity was higher with doxorubicin, while
skin toxicity was more common in PLD arm [21]. Aldoxorubicin
is a new form of albumin-bound doxorubicin, which is designed
with the idea that albumin carries doxorubicin to the more acidic
tumour environment, where the bond is then disconnected. This
way, the drug accumulates in tumour environment, allowing
higher concentrations of doxorubicin with less systemic toxicity
[22]. In a phase IIb randomized multicenter study, aldoxorubicin
was compared to doxorubicin and the results showed improved
progression-free survival in the experimental arm (5.6 [95% CI, 3.0-
8.1] vs. 2.7 [95% CI, 1.6-4.3] months; P = .02). Response rates were
higher in the aldoxorubicin group as well (20 percent vs. 0 percent),
but grade 3 or 4 neutropenia was also more common (29 vs. 12 percent), but not febrile neutropenia [23]. Ifosfamide, a nitrogen
mustard alkylating agent, has response rates of up to 18% as a single
agent therapy [1,24]. It can be used as an alternative to anthracyclines
in cases with contraindications i.e. cardiac failure or intolerance. It
has a more established role as a second line agent, after progression
on anthracyclines. Tumours that develop resistance to standarddose
ifosfamide can be respond to increased dose but at the cost of
increased toxicity [25]. Dacarbazine (DTIC) along with doxorubicin
is one of the oldest drugs to have shown activity in STS. It was first
described as a possible option as a monotherapy in advanced disease,
with reported response rate of 18%; but a relatively short time to
progression [26]. Subsequent trials investigated its use in combination
with Doxorubicin. Borden et al. [20] showed increase in response
rates compared to Doxorubicin alone, in patients with metastatic
disease. With the emergence of Mesna, which enabled administration
of high-dose Ifosfamide as an option for dual therapy, Dacarbazine
has fallen out of favor, and has become reserved for patients who
did not tolerate the side effects of this newer agent. A systematic
review published in 2013 by Sharma et al. [27] investigated its use
as second line therapy with and without Gemcitabine. They showed
a 54.2% rate of disease free progression at 3 months when used as
combination therapy, compared to 35.2% in the single treatment
groups. Gemcitabine is another active agent with a reported response
rate of 18% [28]. Combination of gemcitabine with docetaxel has
shown to increase response rates, even though docetaxel on its own
does not have significant activity in STS. A randomised phase II
study by Maki et al. [29] showed tumour response rates of 8% with
gemcitabine alone compared to 16% with combination therapy of
gemcitabine and docetaxel. Docetaxel as a first line therapy or as a
single second line agent has also been examined in advanced disease
with limited success [30]. In phase III GeDDiS trial, gemcitabine /
docetaxel combination was compared with doxorubicin in the firstline.
The primary end-point, progression-free survival was similar in
both arms (HR 1.28, 95% CI, 0.98–1.67; P = .07) but the combination
arm had more dose delays and patient withdrawals due to toxicity
[31]. The results reaffirmed the role of doxorubicin as preferred firstline
choice in STS. Taxanes in general have not shown action across
most subtypes of STS, hower angiosarcoma seem to be particularly
sensitive to taxanes. Response rates of up to 64 percent have been
reported in a retrospective study published by EORTC soft tissue and
bone sarcoma group [32]. Their activity was confirmed in prospective
phase II ANGIOTAX study, although the response was more modest
(18.5%) [33]. This makes paclitaxel an agent of choice in first-line
treatment of this sarcoma subtype. Newer agents are harbouring
interest including Eribulin, a medication derived from marine
sponges, that acts to inhibit mitosis by blocking production of cellular
microtubules [1]. A phase III trial by Schoffski et al published earlier
this year, compared Eribulin to Dacarbazine in second line treatment
of advanced Liposarcoma and Leiomyosarcoma. They found a
significant overall survival benefit: 13.5 months Eribulin group
compared to 11.5 months in the dacarbazine group. Interestingly,
the survival increase was seen but there was no significant difference
in progression-free survival [34]. Patient with Liposarcoma had the
most significant benefit from treatment, so it has been approved by
the FDA for treatment in this type of sarcoma.
Trabectedin is another active agent that is now used in the
treatment of metastatic and locally advanced Liposarcoma but also
in Leiomyosarcoma [1]. It too was originally derived from marine
aquatica, but from sea tunicates. In a randomized phase III study
this agent was compared to Dacarbazine in patients with pretreated
liposarcoma or Leiomyosarcoma; and found it significantly improved
progression free survival (4.2 months vs. 1.5 months - HR 0.55; p
<0.001). The overall survival difference though was not statistically
significant [35]. Both treatments were well tolerated, with similar rate
of myelosupression but higher frequency of transient elevation of
transaminases in trabectedin arm.
Targeted Therapy
With the success of the tyrosine kinase inhibitor imatinib in treatment of gastrointestinal stromal tumours [36] the attention has shifted to small molecule targeted agents as a potential solution to overcoming resistance of STS to cytotoxic chemotherapy. However, subsequent studies with imatinib did not show equally impressive results in other histological subtypes [37] except for dermatofibrosarcoma protuberans that harbor translocation 17:22, where partial response was reported in 46% of patients [38]. Pazopanib, a potent multitargeted tyrosine-kinase inhibitor was the first treatment that had phase III date published in second line setting for STS. The study excluded adipocytic sarcoma based on negative signal for this subtype in phase II trial, and patients were randomized to pazopanib or placebo, with no subsequent cross-over. The primary end point of progression-free survival was significantly improved in experimental arm (4.6 vs. 1.6 months), but the overall survival difference was not significant (12.5 vs. 10.7 months) [39]. Positive results with pazopanib have ignited interest in using other VEGF inhibitors, with several positive phase II trials reported to date. Cediranib has shown 35% response rate in single arm study of patients with alveolar soft part sarcoma – a highly vascular subtype of sarcoma [40]. Sunitinib was trialed in heterogeneous group of sarcomas, with one partial response out of 48 patients, and disease control rate of 23% at 16 weeks [41]. Olaratumab, a monoclonal antibody which inhibits human Platelet-derived growth factor receptor alpha (PDFGRa), has shown exciting progress in advanced disease. A study published recently published in the Lancet by Tap et al. [39], compared the activity of Doxorubicin in metastatic and locally advanced soft tissue sarcoma to Doxorubicin and Olaratumab. In the phase II part of the trial, participants were randomized to receive doxorubicin 75 mg/m2 with olaratumab or placebo. The primary end point was progression free survival which was 2.5 months longer in experimental arm, but a much more significant difference was noted in overall survival. An 11.8 month difference in the dual therapy group compared to standard chemotherapy regimen [42]. A phase III confirmatory trial has completed recruitment.
Immunotherapy
Immunotherapy has been the latest breakthrough in oncology treatment with remarkable results achieved in treatment of metastatic melanoma with Checkpoint Inhibitors (CPI). CPI are monoclonal antibodies that bind to surface receptors of immune cells and/ or tumor cells and modify immune response of the host to the tumour. Response rates in melanoma in phase III studies were up to 40 percent with PD-1 inhibitors [43] and up to 58 percent with combination CTLA-4 and PD-1 inhibitors [44], and a proportion of the patients achieve ongoing long term disease control. In some subtypes of STS, responses have been reported but overall response rates are disappointing compared to some tumour subtypes. A multicenter phase II study of PD-1 inhibitor pembrolizumab in STS and bone sarcomas presented at 2016 American Society of Clinical Oncology Annual Meeting reported no responses in 29 patients with leiomyosarcoma, liposarcoma and synovial sarcoma, but there were 2/9 patients with undifferentiated pleomorphic sarcoma that had a response [45-49]. However, further studies are ongoing, and numerous new checkpoint inhibitors are currently being tested alone or in combination.
Conclusion
From the above review it can be said that sarcoma diagnosis remains a complex clinical problem, in terms of management as well as addressing research design. Given the heterogeneity of disease presentation and low incidence, the management of potentially curable disease requires involvement of multidisciplinary team, experienced in treating these tumors in high-volume centers. Advanced sarcoma continues to have poor prognosis, with only a minority of patients achieving meaningful benefit from cytotoxic chemotherapy. Encouraging results with targeted agents are offering promise in certain subtypes of sarcoma but more mature data and confirmation studies are awaited. A number of early trials involving targeted and immunotherapy agents are ongoing, and patient enrolment is strongly encouraged in this area of desperate need for effective treatments.
References
- Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. 2016; 122: 2952-2960.
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997; 350: 1647-1654.
- Dickinson IC, Whitwell DJ, Battistuta D. Surgical margin and its influence on survival in soft tissue sarcoma. ANZ J Surg. 2006; 76: 104-109.
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005; 353: 701-711.
- Mann GB, Lewis JJ, Brennan MF. Adult soft tissue sarcoma. Aust N Z J Surg. 1999; 69: 336-343.
- Wilkinson MJ, Martin JL, Khan AA, Hayes AJ, Thomas JM, Strauss DC. Percutaneous core needle biopsy in retroperitoneal sarcomas does not influence local recurrence or overall survival. Ann Surg Oncol. 2015; 22: 853-858.
- Management of primary retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2015; 22; 256-263.
- Loghdey MS, Varma S, Rajpara SM, Al-Rawi H, Perks G, Perkins W. Mohs micrographic surgery for dermatofibrosarcoma protuberans (DFSP): a single-centre series of 76 patients treated by frozen-section Mohs micrographic surgery with a review of the literature. J Plast Reconstr Aesthet Surg. 2014; 67: 1315-1321.
- White JS, Biberdorf D, Difrancesco LM, Kurien E, Temple W. Use of tissue expanders and pre-operative external beam radiotherapy in the treatment of retroperitoneal sarcoma. Ann Surg Oncology. 2007; 14: 583-590.
- Yu JI, Lim do H, Park HC, Nam H, Kim BK, Kim SJ, et al. Clinical outcomes of tissue expanders on adjuvant radiotherapy of resected retroperitoneal sarcoma. Medicine. 2016; 95: e4123.
- Lindberg RD, Martin RG, Romsdahl MM, Barkley HT Jr. Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981; 47: 2391-2397.
- Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008; 113: 573-581.
- Bramwell V, Rouesse J, Steward W, Santoro A, Schraffordt-Koops H, Buesa J, et al. Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma--reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1994; 12: 1137-1149.
- Woll PJ, Reichardt P, Le Cesne A, Bonvalot S, Azzarelli A, Hoekstra HJ, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. The Lancet. Oncology. 2012; 13: 1045-1054.
- Le Cesne A, Ouali M, Leahy MG, Santoro A, Hoekstra HJ, Hohenberger P, et al. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: pooled analysis of two STBSG-EORTC phase III clinical trials. Ann Oncol. 2014; 25: 2425-2432.
- Van Glabbeke M, Van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999; 17: 150-157.
- Bae S, Crowe P, Gowda R. Patterns of care for patients with advanced soft tissue sarcoma: experience from Australian sarcoma services. Clinical sarcoma research. 2016; 6: 11.
- Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I. UK guidelines for the management of soft tissue sarcomas. Clinical sarcoma research. 2016; 6-20.
- Ian J, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Jean-Yves Blay, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. The Lancet. Oncology. 2014; 15: 415-423.
- Borden EC, Amato DA, Rosenbaum C, Enterline HT, Shiraki MJ, Creech RH, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol. 1987; 5: 840-850.
- Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 1990; 37: 870-877.
- Metaxas Y, Oikonomopoulos G, Pentheroudakis G. Update on clinical research and state of the art management of patients with advanced sarcomas and GIST. ESMO open. 2016.
- Chawla SP, Papai Z, Mukhametshina G, Sankhala K, Vasylyev L, Fedenko A, et al. First-Line Aldoxorubicin vs Doxorubicin in Metastatic or Locally Advanced Unresectable Soft-Tissue Sarcoma: A Phase 2b Randomized Clinical Trial. JAMA oncology. 2015; 1: 1272-1280.
- Lee SH, Chang MH, Baek KK, Han B, Lim T, Lee J, et al. High-dose ifosfamide as second- or third-line chemotherapy in refractory bone and soft tissue sarcoma patients. Oncology. 2011; 80: 257-261.
- Le Cesne A, Antoine E, Spielmann M, Le Chevalier T, Brain E, Toussaint C, et al. High-dose ifosfamide: circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol. 1995; 13: 1600-1608.
- Buesa JM, Mouridsen HT, van Oosterom AT, Verweij J, Wagener T, Steward W, et al. High-dose DTIC in advanced soft-tissue sarcomas in the adult. A phase II study of the E.O.R.T.C. Soft Tissue and Bone Sarcoma Group. Ann Oncol. 1991; 2: 307-309.
- Sharma S, Takyar S, Manson SC, Powell S, Penel N. Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: a systematic review. BMC cancer. 2013; 13: 385.
- Sharma S, Takyar S, Manson SC, Powell S, Penel N. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. BMC Cancer. 2001; 19: 3483-3489.
- Maki RG, Wathen JK, Patel SR. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007; 25: 2755-2763.
- Verweij J, Lee SM, Ruka W, Buesa J, Coleman R, van Hoessel R, et al. Randomized phase II study of docetaxel versus doxorubicin in first- and second-line chemotherapy for locally advanced or metastatic soft tissue sarcomas in adults: a study of the european organization for research and treatment of cancer soft tissue and bone sarcoma group. J Clin Oncol. 2000; 18: 2081-2086.
- Seddon BM, Whelan J, Strauss SJ. GeDDiS: a prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas (EudraCT 2009-014907-29). Presented at: ASCO Annual Meeting Proceedings. 2015.
- Schlemmer M, Reichardt P, Verweij J, Hartmann JT, Judson I, Thyss A, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. European journal of cancer (Oxford, England. 1990; 44: 2433-2436.
- Penel N, Bui BN, Bay JO. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008; 26; 5269-5274.
- Schöffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016; 387: 1629-1637.
- Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol. 2016; 34: 786-793.
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347: 472-480.
- Chugh R, Wathen JK, Maki RG, Benjamin RS, Patel SR, Meyers PA, et al. Phase II multicenter trial of imatinib in 10 histologic subtypes of sarcoma using a bayesian hierarchical statistical model. J Clin Oncol. 2009; 27: 3148-3153.
- Rutkowski P, Van Glabbeke M, Rankin CJ, Ruka W, Rubin BP, Debiec-Rychter M, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol. 2010; 28: 1772-1779.
- Van Der Graaf WT, Blay JY, Chawla SP. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012; 379: 1879-1886.
- Kummar S, Allen D, Monks A, Polley EC, Hose CD, Ivy SP, et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol. 2013; 31: 2296-2302.
- George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009; 27: 3154-3160.
- Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016; 388: 488-497.
- Robert C, Long GV, Brady B. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015; 372: 320-330.
- Larkin J, Chiarion-Sileni V, Gonzalez R. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015; 373: 23-34.
- Tawbi Ha-H, Burgess MA, Crowley J. Safety and efficacy of PD-1 blockade using pembrolizumab in patients with advanced soft tissue (STS) and bone sarcomas (BS): Results of SARC028--A multicenter phase II study. Presented at: ASCO Annual Meeting Proceedings. 2016.
- Antman K, Crowley J, Balcerzak SP, Rivkin SE, Weiss GR, Elias A, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. Journal of clinical oncology : J Clin Oncol. 1993; 11: 1276-1285.
- Elias A, Ryan L, Sulkes A, Collins J, Aisner J, Antman KH. Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol. 1989; 7: 1208-1216.
- van Oosterom AT, Mouridsen HT, Nielsen OS, Dombernowsky P, Krzemieniecki K, Judson I, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol. 2011; 29: 2528-2533.
- van Oosterom AT, Mouridsen HT, Nielsen OS, Dombernowsky P, Krzemieniecki K, Judson I, et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer. 1990; 38: 2397-2406.