Case Series
Could Detailed Alk-Status Reporting Help Better Understand Therapy Response to Crizotinib?
Maximilian von Laffert1, Florian Alius2, Ergin Kilic1, Manuela Gerhold1, Dido Lenze1, Albrecht
Stenzinger3, Bettina Temmesfeld-Wollbrück2, Manfred Dietel1, Norbert Suttorp2, Jens-Carsten Rückert4, Jens Neudecker4, Mahmoud Ismail4, Nikolaj Frost2, Michael Hummel1, Antje Tessmer2 and Frederick Klauschen1*
1Department of Pathology, Humboldt University Berlin, Germany
2Department of Internal Medicine/Infectious Diseases, Charité, Medical School, Germany
3Department of Pathology, University of Heidelberg, Germany
4Department of General, Visceral, Vascular and Thoracic Surgery, Universitatsmedizin Berlin, Germany
*Corresponding author: Frederick Klauschen, Department of Pathology, Humboldt University Berlin, Campus Charité Mitte Charité Universitätsmedizin Berlin Charitéplatz 1, 10117 Berlin, Germany
Published: 01 Dec, 2016
Cite this article as: von Laffert M, Alius F, Kilic E, Gerhold
M, Lenze D, Stenzinger A, et al. Could
Detailed Alk-Status Reporting Help
Better Understand Therapy Response
to Crizotinib?. Clin Oncol. 2016; 1:
1152.
Abstract
Anaplastic lymphoma kinase (ALK) rearrangements have been found to be actionable mutations in
non-small cell lung cancer (NSCLC). ALK-tyrosine kinase inhibitors (TKI) showed very promising
therapy response rates and extended progression free survival. We report on three patients, each
with an unequivocal ALK-positive status on the protein and genetic level. Two patients show a
good response to ALK-inhibition whereas one patient is a non-responder. The latter displayed an
unequivocal ALK-positive pattern by immunohistochemistry (IHC) and FISH (fluorescence insitu
hybridization), the latter with an additional copy number gain (CNG) of the 3´signal. To date,
CNG-patterns were reported as contributors to acquired crizotinib resistance only. Despite the
anecdotal character, our report might suggest that this kind of ALK-FISH pattern also plays a role
in the context of intrinsic resistance.
Keywords: Anaplastic lymphoma kinase; Non-small cell lung cancer; Fluorescence in situ hybridization; Immunohistochemistry; Intrinsic resistance; Acquired resistance
Abbreviations
ALK: Anaplastic Lymphoma Kinase; NSCLC: Non-Small Cell Lung Cancer; FISH: Fluorescence in situ hybridization; IHC: Immunohistochemistry
Introduction
Anaplastic Lymphoma Kinase (ALK) rearrangements have been found to be actionable mutations in non-small cell lung cancer (NSCLC) [1-3]. Detectable in approximately 4% of the patients, their occurrence appears to be associated with younger age, light or never smoking as well as adenocarcinoma histology [2-4]. The ALK-tyrosine kinase inhibitor (TKI) crizotinib (and more recently ceritinib and alectinib) showed very promising therapy response rates and extended progression free survival [2,3]. ALK-status needs to be tested prior to therapy which is usually done by fluorescence in-situ hybridization (FISH) and immunohistochemistry (IHC) [5]. Here we report on three patients, each with an unequivocal ALK-positive status on the protein and genetic level. Two patients show a good response to ALK-inhibition whereas one patient is a non-responder. It is known that TKI-resistance cannot only develop during therapy but may exist intrinsically. Here, we put the similarities and differences among the reported cases with respect to ALK-rearrangements into the context of their therapy response and discuss the implications our anecdotal findings may have for ALK-status reporting.
Case Presentation
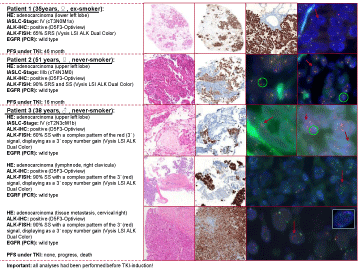
Case 1
Case 1 is a 35-year old woman with a former smoking history (4 pack years, non-smoker since
10 years) and the first diagnosis of a stage IV NSCLC (cT3N0M1a, lower left lobe) in December 2010
and a positive family history with both grandmothers having died of lung cancer. At first, wedgeresection
of the right upper lobe (M1a) was performed to confirm the metastatic stage, followed by
four cycles of cisplatin and pemetrexed (02/2011-05/2011). Subsequently, the regimen was changed to erlotinib (“switch-maintenance”). Under this treatment the patient
showed disease control until 01/2012. Due to progress 02/2012 one
cycle of pemetrexed was administered. Concurrent ALK-testing
revealed positivity on the immunohistochemical (D5F3-Optiview) [6]
and molecular level (Vysis LSI ALK Dual Color, Abbott Molecular,
Abbott Park, IL) [7]. The latter (FISH) showed single red signals
(SRS) in approximately 65% of the tumor cells. As a consequence,
therapy with crizotinib was started in 02/2012 (compassionate use
program). Already in 04/2012 a partial remission was noted. This
regimen was conducted for a total of 22 month with partial remission
and no signs of progress. In December 2013 pulmonary progress
and a new lesion in the left kidney were noted and the patient was
enrolled in the second line TKI study with alectinib under which a
partial response has been reached after 8 weeks. Until to date there
was no tumor progress (total survival after TKI: 46 month) (Figure 1).
Case 2
Case 2 is a 51-year old woman with a never-smoking history and
the first diagnosis of a stage IIIB NSCLC (cT4N3M0, upper left lobe)
in October 2011. ALK-testing revealed strong protein expression
(D5F3-Optiview) and single red signals (SRS), as well as some split
signals (SS) in FISH-analysis (approximately 65% of the tumor cells).
Initially, the patient was treated with first-line cisplatin/vinorelbin but
experienced disease progression after four cycles of therapy (10/2011-
01/2012). Therefore, the regimen was changed to pemetrexed
(02/2012-08/2012) under which the disease was initially stable,
however, showed progress after eight cycles and was then switched
to docetaxel (four cycles, until 10/2012). Instantly after crizotinib
approval in Europe, therapy was initiated due to tumor progress.
Unfortunately, multiple brain metastasis (<1cm) were detected at the same time and consecutively treated with whole brain radiation
(10 x 3Gy). The primary tumor mass in the lung already showed
partial remission after two month of therapy. In the following the
patient showed stable disease for another 11 month. In October 2013,
progression was noted, due to aggravating cough, pleura effusions, as
well as new brain metastasis. Therefore the patient was also enrolled
in a study with the second line TKI alectinib under which a partial
remission (brain and lung) has been reached with a stable disease
until April 2014. However, due to tumor progress the patient died in
July 2014 (total survival after TKI: 16 month).
Case 3
Case 3 was a 38-year old never-smoking man with the first
diagnosis of a stage IV NSCLC (cT2cN3cM1b, upper left lobe) in
April 2012 (metastasis in lung, liver, bones and brain). First line
therapy was performed with cisplatin/vinorelbin and carboplatin/
vinorelbin for two cycles each (05/2012-07/2012) and was followed by
a switch-maintenance therapy with pemetrexed for 3 cycles. Tumor
progress and positive ALK-testing (methods as described above)
led to the induction of crizotinib in November 2012, but tumor and
metastasis still showed rapid progress. After two month of therapy a
fourth line approach (docetaxel) was tried, however, unfortunately
the patient died in February 2013. ALK-tumor-analyses at three
different sites (primary cancer, lymph node and tissue metastasis)
revealed consistent results for IHC and FISH respectively. The latter
displayed a SS-pattern with an additional copy number gain (CNG)
of the 3´signal (red).
Figure 1
Figure 1
Clinical and morphological (H&E, ALK-IHC, ALK-FISH) data of three ALK-positive patients before crizotinib therapy (EGFR: epidermal growth factor
receptor, FISH: fluorescence in-situ hybridization, IHC: immunohistochemistry, PFS: progression free survival, SRS: single red signal, SS: split signal, TKI: tyrosine
kinase inhibitor).
Discussion
(a) We present three patients with ALK-positivity by means of IHC and FISH but with different therapy outcome. While two patients
showed encouraging therapy response rates comparable to already
published data [2,3,8,9], one patient did not benefit from this therapy
regimen at all. Progression under therapy is a well-documented
phenomenon; however, in most cases it occurs after several months
or even years. This so-called acquired resistance is believed to be due
to several mechanisms, [10-12] e.additional secondary mutations in
the kinase of the EML4-ALK gene
(b) copy number gain of the ALK gene
(c) additional secondary EGFR mutation
(d) emergence of an ALK gene fusion negative tumor, no
alternate driver
(e) secondary KRAS mutations
Furthermore, EGFR and KRAS mutations had also been described
in ALK-rearranged tumor samples of crizotinib-naïve patients [11]
and are discussed as contributors of intrinsic resistance as well [10,11].
The latter findings could explain reports of patients with minimal
therapy benefit (e.g. two weeks only!), however, detailed molecular
analyses were not performed in those cases [3,8]. In this report, we
evaluated and compared the pre-crizotinib ALK-FISH status of three
patients. Interestingly, the three different tumor regions evaluated
in the non-responding patient revealed an uncommon pattern. We
observed split signals with an additional copy number gain of the
3´part of the ALK-split (red signal). To date, CNG-patterns were
reported as contributors to acquired crizotinib resistance only [10-
12]. Despite the anecdotal character, our report might suggest that
this kind of ALK-FISH pattern also plays a role in the context of
intrinsic resistance. This will have to be investigated in larger studies.
To conclude, our report points to the potentially important role
of a detailed ALK-FISH analysis that should also appreciate subtle
patterns (besides well-known SS and SRS) even in cases considered
unequivocally ALK-positive according to current guidelines. This
argument is further underlined by recent case reports describing
atypical or putative negative ALK-FISH patterns in patients, who
were selected for crizotinib therapy based on IHC and/or PCR results
and showed therapy response [13,14].
References
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448: 561-566.
- Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011; 12: 1004-1012.
- Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363: 1693-1703.
- Dragnev KH, Gehr G, Memoli VA, Tafe LJ. ALK-Rearranged Adenosquamous Lung Cancer Presenting as Squamous Cell Carcinoma: A Potential Challenge to Histologic Type Triaging of NSCLC Biopsies for Molecular Studies. Clin Lung Cancer. 2014; 15: e37-e40.
- Sholl LM, Weremowicz S, Gray SW, Wong KK, Chirieac LR, Lindeman NI, et al. Combined Use of ALK Immunohistochemistry and FISH for Optimal Detection of ALK-Rearranged Lung Adenocarcinomas. J Thorac Oncol. 2013; 8: 322-328.
- Nitta H, Tsuta K, Yoshida A, Ho SN, Kelly BD, Murata LB, et al. New methods for ALK status diagnosis in non- small-cell lung cancer: an improved ALK immunohistochemical assay and a new, Brightfield, dual ALK IHC-in situ hybridization assay. J Thorac Oncol. 2013; 8: 1019-1031.
- Sholl LM, Weremowicz S, Gray SW, Wong KK, Chirieac LR, Lindeman NI, et al. Combined Use of ALK Immunohistochemistry and FISH for Optimal Detection of ALK-Rearranged Lung Adenocarcinomas. J Thorac Oncol. 2013; 8:322-328.
- Helen Tufman AM, Edelmann M, Gamarra F, Reu S, Borgmeier A, Schrödl K, et al. Preselection based on clinical characteristics in german non-small-cell lung cancer patients screened for EML4-ALK translocation. J Thorac Oncol. 2014; 9: 109-113.
- Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2013; 8: 1570-1573.
- Tibaldi C. Research Highlights: Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small-cell lung cancer. Pharmacogenomics. 2014; 15: 133-135.
- Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012; 18: 1472-1482.
- Doebele RC. A Nice Problem to Have: When ALK Inhibitor Therapy Works Better Than Expected. J Thorac Oncol. 2014; 9: 433-435.
- Ren S, Hirsch FR, Varella-Garcia M, Aisner DL, Boyle T, Zhou C, et al. Atypical Negative ALK Break-Apart FISH Harboring a Crizotinib-Responsive ALK Rearrangement in Non-Small-Cell Lung Cancer. J Thorac Oncol. 2014; 9: e21-e23.
- Rosoux A, Pauwels P, Duplaquet F, D'Haene N, Weynand B, Delos M, et al. Effectiveness of crizotinib in a patient with ALK IHC-positive/FISH-negative metastatic lung adenocarcinoma. Lung Cancer. 2016; 98: 118-121.