Case Report
Complete Pathologic Response of Pancreatic Adenocarcinoma to Chemotherapy (FOLFIRINOX) is not equal to Cure: Case Report and Review of the Literature
Gioia Pozza1, Alice Tonello1, Giuseppe Patanè1, Isabella Paladina2, Michele Valmasoni1, Stefano Merigliano1 and Cosimo Sperti1*
1Department of Surgery, Oncology and Gastroenterology, University of Padua, Italy
2Department of Oncology, Veneto Institute of Oncology (IOV), Italy
*Corresponding author: CosimoSperti, Department of Surgery, Oncology and Gastroenterology, 3rd Surgical Clinic, via Giustiniani 2, 35128 Padua, Italy
Published: 28 Nov, 2016
Cite this article as: Pozza G, Tonello A, Patanè G, Paladina
I, Valmasoni M, Merigliano S, et
al. Complete Pathologic Response
of Pancreatic Adenocarcinoma to
Chemotherapy (Folfirinox) is not equal
to Cure: Case Report and Review of the
Literature. Clin Oncol. 2016; 1: 1146.
Abstract
Pancreatic ductal adenocarcinoma may present as locally advanced disease in a significant percentage
of patients. Recently, the potent FOLFIRINOX regimen was shown to significantly prolong survival
in advancedpancreatic ductal adenocarcinoma. We herein report a case of complete tumor response
after FOLFIRINOX treatment of alocally advanced pancreatic cancer that was successfully resected.
A 68-year-old woman was hospitalized in July 2014, for a 2-months history of right abdominal
pain and weight loss. Abdominal US and CT showed a 3 cm mass involving the uncinated process
of the pancreas and the retroperitoneal tissue with a pathologically enlarged left para-aortic nodes
until iliac artery bifurcation. Ca 19-9 levels were 159.2 U/mL (normal value <37 U/mL). US-guided
percutaneous biopsy of the mass showed poorly differentiated adenocarcinoma of the pancreas, and
the patient was referred to neo-adjuvant therapy with FOLFIRINOX (6 cycles). Ca 19-9 serum levels
normalized and CT examination showed reduction of the tumor (2.3 cm) together with para-aortic
lymph nodes (<1 cm). 18-FDG PET/CT did not show any pathological uptake of the radiotracer. In
December 2014, the patient underwent pylorus-preserving pancreaticoduodenectomy. Pathological
examination did not show residual cancer cells, and no adjuvant therapy was administered. Sixteen
months after surgery, brain metastasis occurred in absence of other sites of recurrence. Pathological
examination of resected specimen confirmed brain metastasis from pancreatic adenocarcinoma.
One month later, CT-scan showed multiple brain metastases, treated with palliative stereotactic
radiotherapy. Currently, the patient is alive 22 months after pancreatic resection.
Although rare, complete pathologic response of pancreatic adenocarcinoma after neoadjuvant
therapy may occur, but this does not mean cure because tumor’s recurrence may happen.
Keywords: FOLFIRINOX; Neoadjuvant chemotherapy; Pancreas; Pancreatic cancer; Pathological response
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in
the United States. It still is one of the most rapidly progressive and deadly malignancies worldwide
[1] because of the marked tumour resistance to treatment,and the lack of specific early symptoms
resulting in advanced stageat diagnosis [2].
The management of patients with pancreatic carcinoma depends on the stage of the disease.
Surgical resection followed by adjuvant therapy is the standard of care for patients diagnosed with
resectable disease. However, at diagnosis, only 20% of patients fulfill the resectability criteria. At
clinical presentation, 30-40% of patients are affected by a locally advanced cancer due to the abutment
of the celiac axis, aortic invasion, superior mesenteric artery encasement [3] or retroperitoneal
involvement.
In spite of neoadjuvant treatments (chemotherapy- or chemoradiation therapy) are advocated
for locally advanced PDAC,no randomized phase III trials have been carried out to confirm evident
clinical benefit [4]. The randomized phase III PRODIGE trial evaluated FOLFIRINOX versus
gemcitabine alone in patients with metastatic pancreatic cancer and good performance status: a
dramatic improvement in both median progression-free survival and median overall survival in favour of the group receiving FOLFIRINOX was seen [5]. However,
complete pathological response after chemotherapy in advanced
pancreatic cancer is a very rare event. We report a case of a complete
pathological response after FOLFIRINOX treatment in a patient with
advanced pancreatic adenocarcinoma, who subsequently experienced
brain metastases.
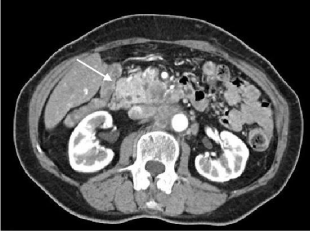
Figure 1
Figure 1
Abdominal CT showing pancreatic head mass (arrow),
retroperitoneal infiltration and enlarged para-aortic nodes.
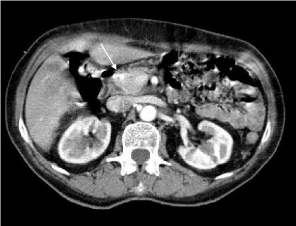
Figure 2
Figure 2
Abdominal CT showing reduction of the tumor (2.3 cm) (arrow) and
of para-aortic nodes (< 1 cm).
Case Presentation
A 68-year-old woman with a 2-months history of right abdominal pain and weight loss underwent abdominal ultrasonography that revealed a solid mass in the uncinated process of the pancreas. Abdominal computed tomography (CT) confirmed a 3 x 2 cm mass in the uncinated process, infiltrating the posterior fat planes, with pathologic, enlarged para-aortic nodes until iliac artery bifurcation (Figure 1). The patient was referred to our Institution in July 2014. Routine laboratory examinations were in the normal range; a mild increase of the tumor marker Ca 19-9 was observed (144 U/mL, normal value <37 UI/mL). Percutaneous US-guided biopsy of the lesion showed a poorly differentiated adenocarcinoma. According to these results, the patient underwent neoadjuvant therapy consisting in 5 cycles of chemotherapy with FOLFIRINOX regimen (irinotecan 180 mg/m² on day 1 , oxaliplatin 85 mg/m² on day 1 , leucovorin 400 mg /m² on day 1 and 5 - fluorouracil 2400 mg /m² continuous infusion over 46 hours every 15 days) from August to October 2014. No dose reduction was required. Major toxicity during the whole treatment was asymptomatic neutropenia G3. Restaging CT-scan showed reduction of pancreatic tumor (23 x 12 mm) and of paraaortic lymph-nodes (<1 cm) (Figure 2), 18-FDG positron emission tomography (PET) did not show pathologic uptake of the radiotracer. Serum Ca 19-9 levels dropped to 4.4 U/mL). Based on radiological down-staging, surgical resection was planned. At laparotomy, in December 2014, no metastatic lesions or major vessels invasion were found. A relevant grade of fibrosis was observed during the dissection. Frozen section examination of omental and para-aortic nodes were negative. So, pylorus-preserving pancreaticoduodenectomy was performed. The post-operative course was uneventful and the patient was discharged on post-operative day 9. The pathological staging was pT0N0M0 with absence of neoplastic cells both in the specimen and in all examined lymph nodes. No adjuvant treatment was performed. Sixteen months after surgery, the patient showed lapses of memory, apraxia, gait imbalance and weakness in the right leg. Brain CT showed a hypodense, 23-mm lesion with surrounding oedema in the frontal region, suggesting a metastatic lesion (Figure 3). CT scan of the thorax and abdomen did not show signs of recurrent disease, and the patient underwent excision of the cerebral mass. Pathological examination revealed poorly differentiated adenocarcinoma consistent with metastasis from a primary pancreatic carcinoma. One month after surgery, brain CT showed multiple metastatic nodules, and stereotactic radiotherapy was started. Thoracic and abdominal CT-scan examination does not show signs of local or other sites of recurrence. The patient is currently alive 22 months after pancreatic resection.
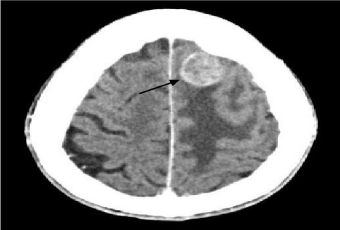
Figure 3
Figure 3
Cerebral CT scan showing a 2.6 cm metastatic localization in the
frontal left lobe (arrow).
Discussion
We presented a very uncommon case of locally advanced
pancreatic cancer (PC) treated with neoadjuvant therapy, that
completely disappeared at pathological examination of resected
specimen. Unfortunately, tumour recurred in the brain and
progressed despite stereotactic radiotherapy. Neo-adjuvant therapy
(i.e. chemotherapy or chemoradiation) and the type of combination
therapyhave been suggested for locally advanced PC even if its role in
clinical practice remains unclear [6].
A new era in the treatment of advanced PC was born in 2003,
when an open phase I study on the feasibility of a combination
therapy consisting of 5-FU/leucovorin plus oxaliplatin and irinotecan
(FOLFIRINOX) for the treatment of patients with metastatic solid
tumors was published [7]. The study showed antitumor activity in two
patients with PC. Later II phase trial specifically addressed patients
with advanced and metastatic PC, showing promising results [8].
The randomized phase III PRODIGE trial evaluated FOLFIRINOX
versus gemcitabine alone in patients with metastatic PC and good
performance status: a dramatic improvement in both median progression-free survival and median overall survival in favour of the
group receiving FOLFIRINOX was seen [9].
Radiotherapy approaches, with or without chemotherapy, have
been frequently used in this subset of patients [10]. A meta-analysis
including 11 studies with 794 patients with locally advanced PC,
examined the use of radio-therapeutic approaches and demonstrated
that overall survival was better with chemoradiation compared to
radiotherapy alone [11].
Due to its efficacy in advanced PC, FOLFIRINOX regimen has
been tested in the neoadjuvant setting of locally advanced, nonmetastatic
adenocarcinoma.
Preliminary results in small groups of patients with border-line
resectablePC showed that the treatment was safe and resulted in a
67% resection rate and a median survival time of 22 months (12). A
recent systematic review of Rombouts et al. [13] collected fourteen
studies with 365 patients treated with FOLFIRINOX; 57% of the
patients (n=208) underwent also radiotherapy. Resectability rate was
28%, R0 resection rate was 77% and overall median survival time
ranged from 8.9 to 25.0 months.
Two meta-analysis of FOLFIRINOX-based neoadjuvant therapy
for locally advanced PC have been published in the last 2 years
[14,15]. Petrelli et al. [14] collected 13 studies for a total of 253
patients. After treatment, 43% of patients underwent resection with
39.4% of R0 resections. Median survival time was reported in only 3
studies and ranged from 13.7 and 24.2 months). The meta-analysis of
Suker et al. [15] included 11 studies and reported survival outcome of
315 patients. Resectability rate ranged from 0% to 43%, R0 resection
ranged from 50% to 100%, and median survival time was 24.2 months.
A large, single centre experience of FOLFIRINOX regimen in
locally advanced PC reported a resectability rate of 50.8% (292/575
patients) with a median survival time of 15.3 months [16].
Despite the retrospective nature of the studies, these data confirm
that FOLFIRINOX therapy is a promising regimen for locally
advanced adenocarcinoma of the pancreas, but further prospective
studies are needed in order to evaluate long-term outcome of these
patients. Complete pathological response after FOLFIRINOX
therapy is infrequent. Rombouts et al. [13] found 6 complete
pathologic responses in 85 (7%) resected specimens, but outcome of
these patients is not reported. Gostimir et al. [17] described a case
of patient treated with FOLFIRINOX for border-line resectable PC.
After pancreaticoduodenectomy, final pathological analysis revealed
no evidence of residual adenocarcinoma, and the patient is alive and
disease-free 15 months after surgery. Hartlapp et al. [18] reported
a case of complete pathological response of a locally advanced PC
treated with 4 cycles of FOLFIRINOX. After resection, adjuvant
chemotherapy with nab-paclitaxel/gemcitabine was administrated
for three months. The patient is disease free 25 months after initial
diagnosis. Turner et al. [19] described the case of a complete
radiological response with chemotherapy and radiotherapy of a
locally advanced PDAC; 6 months after surgery a CT scan showed no
signs of recurrence.
Valeri et al. [20] reported a case of complete pathological response
after 8 cycles of FOLFIRINOX and the patient is alive and without
evidence of disease 14 months after surgery.
Brain metastasis after resection for PC as single site of recurrence
is very uncommon. In 2004, El Kamar et al. [21] reported a patient
with stage IV PC who developed brain metastases and collected 40
similar cases from the Literature: only 13 of which were diagnosed
ante-mortem. More recently, Japanese Authors reported a case of
patient with stage IV PC who initially responded to FOLFIRINOX
therapy, but subsequently progressed with multiple brain metastases
and died 7 months after the first diagnosis [22].
Our patient showed a complete pathologic response to
FOLFIRINOX, but 16 months after pancreaticoduodenectomy brain
metastasis occurred. Despite resection of recurrence, multiple brain
metastases reappeared. To our knowledge, this is the first case of a
single brain recurrence after pancreatic resection and complete
disappearance of primary tumor.
In conclusion, FOLFIRINOX neoadjuvant therapy for locally
advanced PC may allow resection rate in a significant percentage of
patients. Complete pathologic response is a rare event and does not
mean “cure”. More studies and more informations about recurrence
details and survival are needed.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014; 64: 9–29.
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010; 7: 163-172.
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010; 362: 1605-1617.
- Gillen S, Schuster T, Meyer ZumBüschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010; 7: e1000267.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364: 1817-1825.
- White R, Lee C, Anscher M, Gottfried M, Wolff R, Keogan M, et al. Preoperative Chemoradiation for Patients With Locally Advanced Adenocarcinoma of the Pancreas. Ann Surg Oncol. 1999; 6: 38-45.
- Ychou M, Conroy T, Seitz JF, Gourgou S, Hua A, Mery-Mignard D, et al. An open phase I study assessing the feasibility of the triple combination: oxaliplatin plus irinotecan plus leucovorin/ 5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2003; 14: 481-489.
- Conroy T, Paillot B, François E, Bugat R, Jacob JH, Stein U, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a GroupeTumeurs Digestives of the Federation Nationale des Centres de LutteContre le Cancer study. J Clin Oncol. 2005; 23: 1228-1236.
- Ychou M, Desseigne F, Guimbaud R, Ducreux M, Bouché O, Bécouarn Y, et al. Randomized phase II trial comparing folfirinox (5FU/leucovorin [LV], irinotecan [I] and oxaliplatin [O]) vs gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA). First results of the ACCORD 11 trial. J Clin Oncol. 2007; 25: 4516.
- Maheshwari V, Moser AJ. Current management of locally advanced pancreatic cancer. Nat Clin Pract Gastroenterol Hepatol. 2005; 2: 356-364.
- Sultana A, Tudur Smith C, Cunningham D, Starling N, Neoptolemos J, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007; 25: 2607–2615.
- Christians KK, Tsai S, Mahmoud A, Ritch P, Thomas JP, Wiebe L, Kelly T et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm?. Oncologist. 2014; 19: 266-274.
- Rombouts SJ, Walma MS, Vogel JA, van Rijssen LB, Wilmink JW, Mohammad NH, et al. Systematic Review of Resection Rates and Clinical Outcomes After FOLFIRINOX-Based Treatment in Patients with Locally Advanced Pancreatic Cancer. Ann Surg Oncol. 2016.
- Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015; 44: 515-521.
- Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016; 17: 801-810.
- Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg. 2016; 264: 457-463.
- Gostimir M, Bennett S, Moyana T, Sekhon H, Martel G. Complete pathological response following neoadjuvant FOLFIRINOX in borderline resectable pancreatic cancer - a case report and review. BMC Cancer. 2016; 16: 786.
- Hartlapp I, Müller J, Kenn W, Steger U, Isbert C, Scheurlen M, et al. Complete pathological remission of locally advanced, unresectable pancreatic cancer (LAPC) after intensified neoadjuvant chemotherapy. Onkologie. 2013; 36: 123-125.
- Turner K, Levi Sandri GB, Boucher E, Hénno S, Le Prisé E, Meunier B, et al. Complete radiological response of an initially locally advanced unresectable pancreatic cancer to chemoradiotherapy using FOLFIRINOX regimen: report of a case. Clin Res Hepatol Gastroenterol, 2015; 39: e29-e31.
- Valeri S, Borzomati D, Nappo G, Perrone G, Santini D, Coppola R. Complete pathological response after FOLFIRINOX for locally advanced pancreatic cancer. The beginning of a new era? Case report and review of the literature. Pancreatology. 2014; 14: 425-430.
- El Kamar FG, Jindal K, Grossbard ML, Mizrachi HH, Kozuch PS. Pancreatic carcinoma with brain metastases: case report and literature review. Dig Liver Dis. 2004; 36: 355-360.
- Miura T, Nakamura N, Kurihara Y, Yonekura K, Watanabe Y, Sanada T, et al. A Case of Brain Metastases from Pancreatic Cancer That Had Been Treated Effectively with FOLFIRINOX. Gan To Kagaku Ryoho 2015; 42: 2357-2359.