Review Article
Ubiquitin-Conjugating Enzyme E2S (UBE2S), A Potential Tumor Therapeutic Target
Jin Cao and Yue-Zu Fan*
Department of General Surgery, Tongji Hospital, Tongji University, China
*Corresponding author: Yue-Zu Fan, Department of General Surgery, Tongji Hospital, Tongji University School of Medicine, Tongji University, Shanghai, 200065, China
Published: 27 Oct, 2016
Cite this article as: Cao J, Fan Y-Z. Ubiquitin-Conjugating
Enzyme E2S (UBE2S), A Potential
Tumor Therapeutic Target. Clin Oncol.
2016; 1: 1132.
Abstract
Objective: The ubiquitin-proteasome system plays important roles in the control of numerous
processes including cell-cycle, signal transduction, transcriptional regulation, receptor down
regulation, and endocytosis. Ubiquitination is a reversible biochemical process mediated or
participated by ubiquitin activating enzyme (E1), ubiquitin-conjugating enzyme (E2), ubiquitin
ligase (E3), and 26s proteasome. Dysfunction in several ubiquitin-mediated processes has been
shown to cause pathological conditions including malignant transformation. This paper reviews
Ubiquitin-conjugating enzyme E2S (UBE2S) as a potential tumor therapeutic target.
Methods: In this study, we review the expression of UBE2S in some malignant tumors, its influence
on the malignant biological behaviors of cancer and the possible molecular mechanisms in cancer
development, according to the related literatures and our recent studies.
Results: A growing body of evidence suggests ubiquitination is associated with the tumorigenesis
and development of cancer. Recent studies have shown that UBE2S is overexpressed in some
malignant tumors such as breast cancer, esophageal cancer, kidney cancer, cervical cancer and
gallbladder cancer; and was found associated with the malignant biological behaviors including
proliferation, invasion and metastasis of cancer. The related signaling pathways and the possible
molecular mechanisms in cancer development were also shown.
Conclusions: UBE2S is not only overexpressed in some malignant tumors, but also play a crucial
role in tumor growth, invasion and metastasis, thus may be a potential tumor therapeutic target.
Keywords: Ubiquitination; Ubiquitin-conjugating enzyme; Ubiquitin-conjugating enzyme E2S; Cancer
Introduction
A growing body of evidence suggests ubiquitination is associated with the tumorigenesis and
development of cancer. Recent studies have shown that ubiquitin-conjugating enzyme E2S (UBE2S)
is overexpressed in some malignant tumors such as breast cancer, esophageal cancer, kidney cancer
and cervical cancer; and was found associated with the malignant biological behaviors including
proliferation, invasion and metastasis of cancer. In this paper, we review the expression of UBE2S
in some malignant tumors, its influence on the development of cancer and the possible molecular
mechanisms.
Ubiquitin-proteasome system and UBE2S
Ubiquitin is a thermal stability protein composed of 76 amino acid residues, with a kind of
molecular weight of 8.5 kD, and the founding member of the ubiquitin protein family of structurally
conserved protein that regulate a host of processes in eukaryotic cells, mediates selective proteolysis
through its enzymatic conjugation to proteins that contain primary degradation signals, thereby
marking such proteins for degradation by the 26S proteasome, an ATP-dependant multi-subunit
protease [1,2]. Many studies have showed that the Ubiquitin Proteasome System (UPS) plays
important roles in the control of numerous processes, including cell-cycle progression, signal
transduction, transcriptional regulation, receptor downregulation, and endocytosis. Ubiquitination
is a reversible biochemical process mediated or participated by ubiquitin activating enzyme (E1),
ubiquitin-conjugating enzyme (E2), ubiquitin ligase (E3), and 26s or 30s proteasome that attaches
ubiquitin to substrate proteins in order to regulate above multiple cellular functions in UPS [3]. The
process of ubiquitination usually involves three steps (Figure 1): firstly, the C-terminal Gly residue
of ubiquitin is activated in an ATP-requiring step by E1 (Step 1); secondly, activated ubiquitin is next
transferred to an active site Cys residue of a ubiquitin-carrier protein, E2 (Step 2); lastly, catalyzed
by a ubiquitin protein ligase, E3, ubiquitin is linked by its C-terminus
to the substrate protein’s Lys residues (Step 3). Multiple rounds of
ubiquitination result in substrate polyubiquitination that can target
proteins for proteasome-dependent destruction [3,4]. Dysfunction
in several ubiquitin-mediated processes has been shown to cause
pathological conditions including malignant transformation [3].
A growing body of evidence has demonstrated that ubiquitination
is associated with the progression of cancer [5]. There are more than
600 E3 ligases in humans, some of which regulate the expression of
tumor-suppressor or tumor-promoting proteins. For example, p53
is one of the most frequently mutated genes in cancer, and reduced
levels of p53 protein can promote cancer initiation. Mdm2 is an E3
ligase for p53 and is one of the major regulators of p53 expression
[6,7]. Overexpression of Mdm2 is observed in a variety of cancers,
such as breast cancer, oral squamous cell cancer, glioma, lymphoma,
and leukemia [8]. COP1 and Pirh1 are also E3 ligases for p53, and
are overexpressed in several cancers [9-13]. In addition to E3 ligases,
E2-conjugating enzymes are associated with cancer progression [14].
One of the most studied E2s is UBE2C because of its association with
cancer. UBE2C is required for the degradation of mitotic regulators
in cooperation with anaphase-promoting complex/cyclosome
(APC/C) [15,16]. High expression of UBE2C is found in many
human cancers of the brain, lung, cervix, colon, liver, thyroid, breast,
and nasopharynx [17-20]. Depletion of UBE2C from cancer cells
significantly reduced the proliferation of cancer cells and induced
cell apoptosis. UBE2C-overexpressed transgenic mice were prone
to develop into the carcinogen-induced lung cancers and a variety
of spontaneous tumor [21]. These results indicate that UBE2C is
involved in cancer development and progression [22].
Ubiquitin-conjugating enzyme E2S (UBE2S) is a 24-kDa protein
that is a member of the E2 family of ubiquitin-conjugating enzymes,
also known as E2-EPF or E2-EPF Ubiquitin Carrier Protein (UCP),
is essential for the elongation of ubiquitin chains to target substrate
proteins to the 26S proteasome [22,23]. Once UBE2C attaches
ubiquitin onto the target proteins, UBE2S promotes the elongation of
ubiquitin chains for the degradation. Recent studies have shown that
UBE2S is also overexpressed in cancers.
UBE2S’ roles in malignant tumors
UBE2S is highly expressed in a variety of malignant tumors:
High expression of UBE2S was observed in cervical, breast, esophagus
and kidney cancers in the past decade [22-26]. In 2007, Tedesco et
al. identified that E2-EPF mRNA and protein are overexpressed in
breast cancers and is regulated by cell cycle in cervical cancer HeLa
cells, achieving the highest levels during mitosis; and that ER- breast
tumors are more likely to express E2-EPF protein and have detectable
E2-EPF expression in a greater percentage of cells than ER+ tumors,
which is consistent with the presence of E2-EPF in the meta-signature
for undifferentiated cancer because ER- tumors are typically poorly
differentiated and are of higher mitotic grade than ER+ tumors [23].
Ayesha et al. [22] studies also showed that UBE2S is highly expressed
in breast cancer, and is localized to both the nucleus and cytoplasm
in immunostaining and cell fractionation analysis. Thereafter, Liang
et al reported that E2-EPF mRNA was highly expressed in cervical
squamous cancer, and its positive expression was correlated with
clinical tumor stage, i.e., the relative expression level of E2-EPF in
tumors at stage I was significantly lower than the tumors at stage IIIV
[26]. Similarly, Chen et al found that UCP mRNA and protein
were also more highly expressed in human esophagus cancer tissues
than in nonmalignant esophageal specimens, and the increased
UCP was associated with higher expression of HIF-1α, VEGF,
and decreased expression of VHL while the stability of VHL and
HIF-1α are dependent on E2-EPF level [24]. And, Roos et al. [25]
reported that E2-EPF mRNA was significantly elevated in Papillary
Renal Cell Carcinoma (PRCC), in particular type 2 which patients
have poorer prognosis than patients with type 1 PRCC). Recently,
our study by immunohistochemical staining for paraffin specimens
of gallbladder tissues has showed that UBE2S was highly expressed
in gallbladder cancers (n=85) when compared with precancerous
lesions (adenoma and severe dysplasia, n=28; 4.08 ± 3.41 vs. 2.68
± 1.98 score, P=0.0070), benign lesions (inflammation and polyps,
n=15; 4.08 ± 3.41 vs. 2.07 ± 1.33 score, P=0.0002) and normal tissues
adjacent to cancer (n=43; 4.08 ± 3.41 vs. 2.42 ± 1.82 score, P=0.0005)
of the gallbladder; furthermore, western blotting for fresh specimens
of gallbladder cancer (n=10) and normal tissues adjacent to cancer
(n=10) has showed that expression of UBE2S proteins in gallbladder
cancers was higher than that of normal tissues adjacent to cancer
(0.7340 ± 0.1806 vs. 0.1972 ± 0.091, relative grey value; P=0.0020).
And, the gallbladder cancer patients with high expressed UBE2S had
the shortest survival time.
UBE2S associates with proliferation, invasion and metastasis
of malignant tumors: As reported in 2006 by Jung et al. [27], forced
expression of UCP boosts the proliferation, invasion and metastasis
of tumor cells in vitro and in vivo. But Tedesco et al. [23] reported
that reduction of E2-EPF protein levels by >80% using RNAi had no
significant effects on the proliferation of cervical cancer Hela cells or
ER- MDA-MB-231 or MDA-MB-453 breast cancer cells; and thought
that the possible reason was that the 80% to 90% reduction in E2-
EPF levels was not of sufficient magnitude or duration to impact the
proliferation of the cancer cells analyzed or that only certain cancers
are dependent on E2-EPF for their growth. Subsequently, Liang et al.
[26] studies showed that reduction of E2-EPF protein levels by >80%
using shRNA decreased the proliferation, invasion and tumorigenicity
of another cervical cancer cell line SiHa, so supposed that the role of
E2-EPF in cell growth was different depending on the cell type. And,
Ayesha et al. [22] found that UBE2S was associated with malignant
characteristics of breast cancer cells, UBE2S knockdown suppressed
cell spreading, migration, and invasion in three luminal cell lines
(MCF7, T47D, and MDA-MB-453) and three basal cell lines (BT20,
MDA-MB-231, and Hs578t) of breast cancer. Other studies have also
identified that inhibited UCP significantly decrease tumor growth
and the capacity for invasion and metastasis in human esophagus and
renal cancers [24,25]. Recently, our studies for invasive comparison of
high and low aggressive gallbladder cancer cell lines NOZ and SGC996
and microarray analysis for highly aggressive gallbladder cancer NOZ
cells by Affymetrix chip gene expression profile scan have found that
UBE2S is a target gene related to invasion of gallbladder cancers; and
UBE2S knockdown suppressed migration, metastasis and adhesion
capabilities of NOZ cells and EMT process. It is thus concluded that
UBE2S play a crucial role in tumor growth, invasion and metastasis.
UBE2S affects radiotherapy and chemotherapy
Tedesco et al. [23] reported that E2-EPF knockdown sensitized
cervical cancer HeLa cells to the topoisomerase (topo) II inhibitors
etoposide and doxorubicin and also increased topo IIα protein levels,
but had no effect on the sensitivity of Hela cells to chemotherapeutic
agents such as the microtubule stabilizer Taxol or the topoisomerase
I inhibitor camptothecin. But Jing et al. [26] studies found that E2-
EPF knockdown increases the chemosensitivity to topo I inhibitor
topotecan though the mechanism is still unknown, these data suggest
that combined administration of topo I or topo II drugs and E2-EPF
inhibitors may enhance their clinical effectiveness of chemotherapy.
Studies of Liang et al. [26] have demonstrated that overexpression
of UCP was significantly associated with poor response to
neoadjuvant Computerized Controlled Radiation Therapy (CCRT);
and that treatment with UCP silencing vector significantly increase
the sensitivity of esophageal cancer to radiation, rather than cisplatin.
Recently, Hu et al. [28] reported that UBE2S associated with DNA
repair and glioblastoma multiforme resistance to chemotherapy,
and that knockdown of UBE2S expression rendered glioblastoma
cells more sensitive to chemotherapy. Birle et al. [29] reported
that proteasome inhibitor bortezomib decreased tumor Carbonic
Anhydrase IX (CAIX) expression in colon cancer patients; and
identified that the Hypoxia Inducible Factor-1 (HIF-1) response in
cervical cancer xenografts was suppressed by proteasome inhibitor
bortezomib. Together with, UBE2S affects radiotherapy and
chemotherapy for patients with malignant tumor.
UBE2S associates with the prognosis of patients with
malignant tumors: It was showed that negative staining for E2-EPF,
response to neo-adjuvant therapy and tumor stage are significant
prognostic indicators for overall survival in esophageal cancer by
multivariate analysis. And the expression of UCP is associated with
clinical outcome in esophageal cancer, i.e., higher UCP was linked to
worse Overall Survival (OS) and worse Disease Free Survival (DFS)
[25]. In addition, Roos et al observed that PRCC patients with high
E2-EPF expression showed a trend toward increased cancer-related
mortality and disease progression via immunohistochemistry on
tissue microarray and/or Western blot analysis, although a statistical
significance could not be reached mainly due to the limited sample
size [26]. So, it is suggested that E2-EPF might be a molecular
predictor for the prognosis of cancer patients. But, more cases and
more clinical specimens need to be collected and analyzed.
Possible molecular mechanisms of UBE2S in cancer
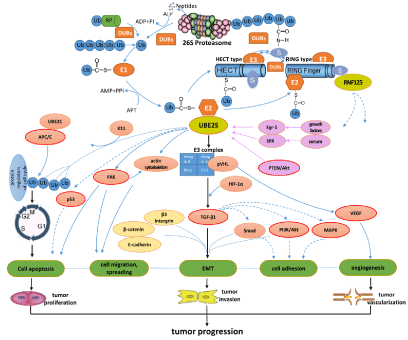
development: As shown in Figure 1, Ubiquitin Proteasome System
(UPS) and its process of ubiquitination of E1, E2 and E3, and some
related signaling pathways of E2 ubiquitin-conjugating enzyme, in
particular UBE2S, to cause tumor progression, i.e. possible molecular
mechanisms of UBE2S in cancer development are illustrated.
UCP–VHL/HIF-1α–TGF-β1–EMT pathway
Maxwell et al. [30] found in 1999 that the von Hippel-Lindau
tumor suppressor (pVHL) targets Hypoxia Inducible Factors (HIFs)
for oxygen-dependent proteolysis. pVHL functions as the substraterecognition
module of the E3 ubiquitin ligase complex composed
of elongin B, elongin C, Rbx1 and Cul2 which ubiquitinates HIF-1α
during normoxia [31-36]; and pVHL is expressed in most tissues
and cell types [37], suggesting that loss of pVHL might be generally
involved in tumor progression and angiogenesis. The heterodimeric
transcription factors HIF-1 and HIF-2, composed of HIF-1α and
HIF-2α and HIF-1β, increase the expression of a number of hypoxia-
inducible genes including the gene encoding Vascular Endothelial
Growth Factor (VEGF), which promotes tumor growth and
vascularization [38,39]. Jung et al. [27] study showed that UCP was
correlated inversely with pVHL in most tumor cell lines, and UCP
was detected coincidently with HIF-1α in human primary liver, colon
and breast cancers, and metastatic cholangiocarcinoma and colon
cancer cells, and also identified for the first time in 2006 that forced
expression of UCP boosts tumor cell proliferation, invasion and
metastasis through effects on the pVHL-HIF pathway. However, they
cannot exclude the possibility that UCP and its cognate E3 may target
other substrates important for cell growth (Figure 1). Thereafter,
Chen et al. [24] proposed that the Epithelial Mesenchymal Transition
(EMT) induced by the VHL/HIF-1α TGF-β1 pathway might be
responsible for the effects regulated by UCP. The reasons are as below:
firstly, TGF-β1 is activated by HIF-1α and plays an important role
in the induction of EMT [40-44], TGF-β1 induces EMT in various
epithelial cell types via PI3K/Akt and SMADs signaling pathway;
secondly, β3 intergrin was reported to facilitate TGF-β-mediated
induction of EMT in epithelial cells of breast and colorectal cancers
[1,2]; thirdly, downregulation of UCP was associated with TGF-β1
and β3 integrin in addition to the decreased HIF-1α and VEGF in
Cuci cells [24]; finally, Rees et al. Reported that TGF-β1 mediates
the EMT associated with loss of β-catenin and E-cadherin, hall
marks of EMT, EMT is a key event in the tumor invasion process
[45]. Consistent with this, Roos et al. [25] reported for the first time
that an inordinately elevated expression of E2-EPF was associated
with decreased pVHL and increased HIF-1α levels in PRCC; and
identified that multiple Hypoxia Responsive Elements (HREs) within
E2-EPF promoter were capable of recruiting HIF1 and HIF2, E2-EPF
transcription is dependent on HIF1.
UBE2S is associated with regulating the actin cytoskeleton
and focal adhesions: Ayesha et al. [22] Reported that UBE2S is
associated with regulating the actin cytoskeleton and focal adhesions.
The depletion of UBE2S induced changes in cellular morphology
and significantly disrupted the formation of actin stress fibers and
focal adhesions while the actin cytoskeleton plays a critical role in
numerous cellular functions such as cell migration and spreading.
It was showed that both migration and spreading were delayed in
the absence of UBE2S. Focal Adhesion Kinase (FAK), except that
it is known to activate survival signals to prevent anoikis, is one of
the most studied protein associates with the regulation of actin
cytoskeletal organization and cell migration, and its inhibitors are
being investigated in clinical trials for cancer treatment [46]. It was
found that the phosphorylation of FAK at Tyr397 was reduced in
the absence of UBE2S. Although the exact molecular mechanisms of
Tyr397 phosphorylation are still not clear, it is believed that integrin
binding to extracellular matrix (ECM) promotes dimerization of FAK
for the phosphorylation of Tyr397 [47,48]. Src family kinases, which
are non-receptor tyrosine kinases, are recruited to the phosphorylated
Tyr397, where they phosphorylate other tyrosine residues of FAK for
the activation of downstream signals [49,50]. Therefore, a decrease
in Tyr397 phosphorylation via UBE2S knockdown may suppress
numerous signals for cell spreading, migration, and invasion. Further
analysis is required to determine whether the functions of FAK or
other signal proteins are affected by UBE2S knockdown to suppress
cell migration and invasion.
Egr-1/SRF–UCP–VHL pathway
E2-EPF Ubiquitin Carrier Protein (UCP) has been shown to
be highly expressed in common human cancers and target von
Hippel-Lindau (VHL) for proteosomal degradation in cells, thereby
stabilizing hypoxia-inducible factor HIF-1α. Lim et al. [51] further
investigated cellular factors that regulate the expression of UCP gene,
and suggesting that growth factors and serum induced Egr-1 and
Serum Response Factor (SRF) can bind the promoter region of E2-
EPF, therefore increase the HIF-1α protein level under non-hypoxic
conditions through the Egr-1/SRF-UCP-VHL pathway.
UBE2S involves in the degradation of protein regulators of
the cell cycle: Ubiquitination by the Anaphase-Promoting Complex
(APC/C) is essential for proliferation in all eukaryotes. Timely
degradation of protein regulators of the cell cycle is essential for the
completion of cell division. UBE2S is involved in the degradation
of proteins by APC/C during mitosis. Once APC/C in combination
with UBE2C primes the lysine residues of substrates with ubiquitin,
UBE2S promotes elongation of ubiquitin chains via K11-mediated
attachment. UBE2S and UBE2C are tightly co-regulated in the cell cycle
by APC/C-dependent degradation; they constitute a physiological
E2-module for APC/C, the activity of which is required for spindle
assembly and cell division. Williamson et al. [52] identified that the
conserved Ube2S as a K11-specific chain elongating E2 for human
and Drosophila APC/C. While depletion of UBE2S already inhibits
APC/C in cells, the loss of the complete UBCH10/UBE2S-module
leads to dramatic stabilization of APC/C substrates, severe spindle
defects and a strong mitotic delay. Garnett et al. [53] reported that the
E2 enzyme UBE2S as an APC/C auxiliary factor that promotes mitotic
exit. Thereafter, Wu et al. [54] found that UBE2S drives elongation
of K11-linked ubiquitin chains by APC, so they propose that UBE2S
is a critical, unique component of the APC ubiquitination pathway.
Recently, studies of Ben-Eliezer et al. [55] identified that appropriate
expression of UBE2C and UBE2S controls the progression of the
first meiotic division. Their depletion reduces the levels of the first
meiotic cytokines is by 50%, and their overexpression doubles and
accelerates its completion (50% as compared with 4% at 11h). It
was also demonstrated that these E2 enzymes take part in ensuring
appropriate spindle formation and also control the extrusion of the
first polar body.
In studies of Whitfield et al. [56], the genome-wide program of
gene expression during the cell division cycle in a human cancer cell
line HeLa was characterized using cDNA microarrays. Transcripts
of >850 genes showed periodic variation during the cell cycle; E2-
EPF was classified as a gene whose expression peaked during the M/
G1 phase of the cell cycle, yet its mRNA expression profile was most
similar to that of the G2/M phase expressed BUB1, BIRC5, CCNB2,
CDC20, PLK1, and other genes involved in mitotic surveillance.
Tedesco et al. [23] also noted that E2-EPF was one of 874 cell cycleregulated
gene identified in gene expression analyses of Hela cells;
however, E2-EPF protein was significantly elevates in S phase as
well as in G2/M phase compared to serum-deprived cell performed
similar cell cycle analyses in HeLa cells. Furthermore, no alteration
in cell cycle distribution was observed in HeLa cells with reduced E2-
EPF expression. Differentiated with this result, recently studies [26]
revealed that E2-EPF knockdown increased the number of cells in the
G0/G1 phase and the percentage of cells in G2/M, especially S phase
are significantly lower in E2-EPF low expression cells than the normal
and control cells.
Other associated pathways
It is newly reported that the stability of UBE2S is regulated by the
PTEN/Akt pathway and that its degradation depends on the ubiquitinproteasome
system. Mechanistically, Akt1 physically interacted with
and phosphorylated UBE2S at Thr 152, enhancing its stability by
inhibiting proteasomal degradation [28]. UBE2S was recently found
to be associated with the components of the Non-Homologous End-
Joining (NHEJ) complex and participated in the NHEJ-mediated
DNA repair process. Knockdown of UBE2S expression inhibited
NHEJ-mediated double- stranded break repair [28].
Figure 1
Figure 1
The possible molecular mechanisms and the related signaling pathways of UBE2S in cancer development.
Ub: Ubiquitin; E1: Ubiquitin-Activating Enzyme; E2: Ubiquitin-Conjugating Enzyme; E3: Ubiquitin Ligase; DUB: Ubiquitin Decomposition Enzyme; UCP: E2-EPF
Ubiquitin Carrier Protein; VHL: von Hippel–Lindau gene; HIF-1α: Hypoxia-Inducible Factor-1α; VEGF: Vascular Endothelial Growth Factor; TGF-β1: Transforming
Growth Factor- β 1; APC/C: Anaphase-Promoting Complex/Cyclosome; FAK: Focal Adhesion Kinase; MAPK: Mitogen-Activated Protein Kinase; PI3K:
Phosphatidylinositol 3-Kinase; EMT: Epithelial- Mesenchymal Transition.
Conclusions
In summary from literature review, we found that UBE2S is highly expressed in a variety of malignant tumors, and associates with tumor stage, neoadjuvant therapy, and prognosis of malignant tumors. In most cases, downregulation or depletion of UBE2S suppressed malignant characteristics such as proliferation, migration, invasion of cancer cells. In addition, these crucial roles of UBE2S were showed to associate to some related signaling pathways such as VHL/ HIF-1α TGF-β1 EMT pathway, etc. UBE2S may be a potential tumor therapeutic target. Targeting these signaling pathways may have potential clinical applications for a variety of malignant tumors.
References
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006; 172: 973-981.
- Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-beta1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006; 66: 9583-9590.
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998. 67: 425-479.
- Varshavsky A. Three decades of studies to understand the functions of the ubiquitin family. Methods Mol Biol. 2012; 832: 1-11.
- Zhou MJ, Chen FZ, Chen HC. Ubiquitination involved enzymes and cancer. Med Oncol. 2014. 31: 93.
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997; 420: 25-27.
- Olson DC, Marechal V, Momand J, Chen J, Romocki C, Levine AJ. Identification and characterization of multiple mdm-2 proteins and mdm-2-p53 protein complexes. Oncogene. 1993; 8: 2353-2360.
- Rayburn E, Zhang R, He J, Wang H. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr Cancer Drug Targets. 2005; 5: 27-41.
- Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003; 112: 779-791.
- Dornan D, Bheddah S, Newton K, Ince W, Frantz GD, Dowd P, et al. COP1, the negative regulator of p53, is overexpressed in breast and ovarian adenocarcinomas. Cancer Res. 2004; 64: 7226-7230.
- Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004; 429: 86-92.
- Duan W, Gao L, Druhan LJ, Zhu WG, Morrison C, Otterson GA, et al. Expression of Pirh2, a newly identified ubiquitin protein ligase, in lung cancer. J Natl Cancer Inst. 2004; 96: 1718-1721.
- Logan IR, Gaughan L, McCracken SR, Sapountzi V, Leung HY, Robson CN. Human PIRH2 enhances androgen receptor signaling through inhibition of histone deacetylase 1 and is overexpressed in prostate cancer. Mol Cell Biol. 2006; 26: 6502-6510.
- Xie C, Powell C, Yao M, Wu J, Dong Q. Ubiquitin-conjugating enzyme E2C: a potential cancer biomarker. Int J Biochem Cell Biol. 2014; 47: 113-117.
- Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008; 133: 653-665.
- Meyer HJ, Rape M. Processive ubiquitin chain formation by the anaphase-promoting complex. Semin Cell Dev Biol. 2011; 22: 544-550.
- Vasiljevic A, Champier J, Figarella-Branger D, Wierinckx A, Jouvet A, Fèvre-Montange M. Molecular characterization of central neurocytomas: potential markers for tumor typing and progression. Neuropathology. 2013; 33: 149-1461.
- Shen Z, Jiang X, Zeng C, Zheng S, Luo B, Zeng Y, et al. High expression of ubiquitin-conjugating enzyme 2C (UBE2C) correlates with nasopharyngeal carcinoma progression. BMC Cancer. 2013; 13: 192.
- Okamoto Y, Ozaki T, Miyazaki K, Aoyama M, Miyazaki M, Nakagawara A. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 2003; 63: 4167-4173.
- Hao Z, Zhang H, Cowell J. Ubiquitin-conjugating enzyme UBE2C: molecular biology, role in tumorigenesis, and potential as a biomarker. Tumour Biol. 2012; 33: 723-730.
- van Ree JH, Jeganathan KB, Malureanu L, van Deursen JM. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J Cell Biol. 2010. 188: 83-100.
- Ayesha AK, Hyodo T, Asano E, Sato N, Mansour MA, Ito S, et al. UBE2S is associated with malignant characteristics of breast cancer cells. Tumour Biol. 2016; 37: 763-772.
- Tedesco D, Zhang J, Trinh L, Lalehzadeh G, Meisner R, Yamaguchi KD, et al. The ubiquitin-conjugating enzyme E2-EPF is overexpressed in primary breast cancer and modulates sensitivity to topoisomerase II inhibition. Neoplasia. 2007; 9: 601-613.
- Chen MF, Lee KD, Lu MS, Chen CC, Hsieh MJ, Liu YH, et al. The predictive role of E2-EPF ubiquitin carrier protein in esophageal squamous cell carcinoma. J Mol Med (Berl). 2009; 87: 307-320.
- Roos FC, Evans AJ, Brenner W, Wondergem B, Klomp J, Heir P, et al. Deregulation of E2-EPF ubiquitin carrier protein in papillary renal cell carcinoma. Am J Pathol. 2011; 178: 853-860.
- Liang J, Nishi H, Bian ML, Higuma C, Sasaki T, Ito H, et al. The ubiquitin-conjugating enzyme E2-EPF is overexpressed in cervical cancer and associates with tumor growth. Oncol Rep. 2012; 28: 1519-1525.
- Jung CR, Hwang KS, Yoo J, Cho WK, Kim JM, Kim WH, et al. E2-EPF UCP targets pVHL for degradation and associates with tumor growth and metastasis. Nat Med. 2006; 12: 809-816.
- Hu L, Li X, Liu Q, Xu J, Ge H, Wang Z, et al. UBE2S, a novel substrate of Akt1, associates with Ku70 and regulates DNA repair and glioblastoma multiforme resistance to chemotherapy. Oncogene. 2016 .
- Birle DC, Hedley DW. Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 2007; 67: 1735-1743.
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999; 399: 271-275.
- Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002; 2: 673-682.
- Koh MY, Darnay BG, Powis G. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1alpha, leading to its oxygen-independent degradation. Mol Cell Biol. 2008; 28: 7081-7095.
- Hägg M, Wennström S. Activation of hypoxia-induced transcription in normoxia. Exp Cell Res. 2005. 306: 180-191.
- Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW, et al. Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A. 1999; 96: 12436-12441.
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000; 2: 423-427.
- Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O, et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999; 284: 657-661.
- Los M, Jansen GH, Kaelin WG, Lips CJ, Blijham GH, Voest EE. Expression pattern of the von Hippel-Lindau protein in human tissues. Lab Invest. 1996; 75: 231-238.
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001; 7: 345-350.
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003; 9: 677-684.
- Gunaratnam L, Morley M, Franovic A, de Paulsen N, Mekhail K, Parolin DA, et al. Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(-/-) renal cell carcinoma cells. J Biol Chem. 2003; 278: 44966-44974.
- Gal A, Sjöblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008; 27: 1218-1230.
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003; 112: 1486-1494.
- Ananth S, Knebelmann B, Grüning W, Dhanabal M, Walz G, Stillman IE, et al. Transforming growth factor beta1 is a target for the von Hippel-Lindau tumor suppressor and a critical growth factor for clear cell renal carcinoma. Cancer Res. 1999; 59: 2210-2216.
- Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007; 117: 3810-3820.
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002; 2: 442-454.
- Bolós V, Gasent JM, López-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010; 3: 83-97.
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994; 14: 1680-1688.
- Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997; 17: 1702-1713.
- Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004; 117: 989-998.
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004; 23: 7928-7946.
- Lim JH, Jung CR, Lee CH, Im DS. Egr-1 and serum response factor are involved in growth factors- and serum-mediated induction of E2-EPF UCP expression that regulates the VHL-HIF pathway. J Cell Biochem. 2008; 105: 1117-1127.
- Williamson A, Wickliffe KE, Mellone BG, Song L, Karpen GH, Rape M. Identification of a physiological E2 module for the human anaphase-promoting complex. Proc Natl Acad Sci U S A. 2009; 106: 18213-18218.
- Garnett MJ, Mansfeld J, Godwin C, Matsusaka T, Wu J, Russell P, et al. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat Cell Biol. 2009; 11: 1363-1369.
- Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A, Kirschner MW. UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci U S A. 2010; 107: 1355-1360.
- Ben-Eliezer I, Pomerantz Y, Galiani D, Nevo N, Dekel N. Appropriate expression of Ube2C and Ube2S controls the progression of the first meiotic division. FASEB J. 2015; 29: 4670-4681.
- Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002; 13: 1977-2000.