Research Article
Delirium Improvement with Mianserin Suppositories in Cancer Patients
Rei Tanaka1*, Hiroshi Ishikawa1, Tetsu Sato1, Michihiro Shino1, Teruaki Matsumoto2, Katsuhiro Omae3 and Iwao Osaka4
1Department of Pharmacy, Shizuoka Cancer Center, Japan
2Department of Psycho-Oncology, Shizuoka Cancer Center, Japan
3Department of Clinical Research, Shizuoka Cancer Center, Japan
4Department of Palliative Medicine, Shizuoka Cancer Center, Japan
*Corresponding author: Rei Tanaka, Departments of Pharmacy, Shizuoka Cancer Center, 1007 Shimonagakubo, Nagaizumi-cho, Sunto-gun, Shizuoka 411-8777, Japan
Published: 21 Oct, 2016
Cite this article as: Tanaka R, Ishikawa H, Sato T, Shino
M, Matsumoto T, Omae K, et al.
Delirium Improvement with Mianserin
Suppositories in Cancer Patients. Clin
Oncol. 2016; 1: 1127.
Abstract
Delirium is a significant problem in palliative medicine, and its proper management and prevention
is extremely important. In our cancer center, mianserin suppositories have been included in the
hospital formulary and are prescribed for cancer patients who have dysphagia; however, previous
studies have shown only limited data on the effect of mianserin suppositories. Therefore, in this
study, we compared the therapeutic effect of mianserin suppositories with mianserin tablets for
improvement of delirium.
Subjects were 66 cancer patients who had received either mianserin suppositories or mianserin
tablets for delirium at our cancer center between April 2013 and March 2015. Delirium was
evaluated using the Intensive Care Delirium Screening Check list (ICDSC). The improvement rates
of delirium for 7 days were compared between the two groups using Fisher’s exact test.
The dose of mianserin was 30-60 mg/day (median dose 30mg/day) in the suppository group (n=31)
and 10-60 mg/day (median dose 20mg/day) in the tablet group (n=35). The improvement rates of
delirium during the 7-day test period were 71.0% in the suppository group and 62.9% in the tablet
group. There was no significant difference. (P=0.60)
Considering the results of this study, mianserin suppositories have an equal delirium improvement
effect compared with mianserin tablets. These findings also suggest that mianserin suppositories can
be effectively used to improve delirium in patients with dysphagia.
Keywords: Delirium; Mianserin; Suppositories; Hospital formulary; Cancer; Palliative care
Introduction
There are mainly 3 types of factors associated with delirium: direct causative factors such as
encephalopathy and use of a psychotropic agent; predisposing factors, such as underlying physical
conditions (e.g. age ≥60 years); and precipitating factors, such as stress and sleeping disorders,
which aggravate the condition or help the condition to persist [1]. Cancer patients often have all
3 types of factors, and are likely to be at high risk of developing delirium. Thus, management and
prevention of delirium are extremely important in care for patients with cancer. In addition to the
removing these factors, conservative therapy with haloperidol [2,3], risperidone [4], lorazepam [5] and mianserin [6-8] were reported to be effective in the treatment of delirium.
Although the mechanism of action of mianserin for treating delirium remains unclear [9], it is
a useful alternative to haloperidol, because of its milder antidopaminergic action and consequent
lower risk of extra pyramidal side effects [10,11]. Mianserin is currently available only asoral tablets.
However, the oral route is often unavailable in cancer patients with delirium, indicating the need for
mianserin in suppository or injectable formulations for treating such patients. Our cancer treatment
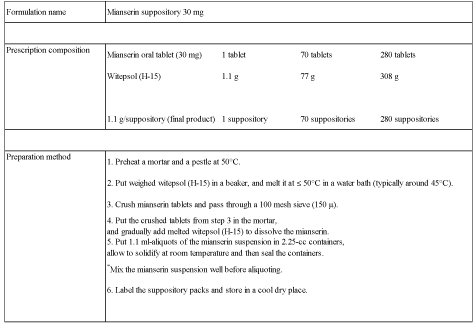
center includes 30mg mianserin suppositories in the hospital formulary for treatment of delirium
(Figure 1), and these are used for treatment of delirium in cancer patients who have difficulty in
taking oral medications. Because an anti-delirium agent needs to be administered for several days
in cancer patients, mianserin suppositories are advantageous with their simple noninvasive route of
administration, compared to haloperidol which is administered by intravenous infusion. Although
benzodiazepines are available as suppositories, the therapeutic effect in delirium is reported to be
unsatisfactory [3]. Furthermore, there are associated risks of adverse
events (e.g., aggravation of disease condition and over sedation)
during treatment and upon withdrawal [12]. In clear contrast to
benzodiazepines, mianserin suppositories are not associated with
any risk of such adverse events, suggesting that they are readily
usable in the treatment of delirium. The drug release profile and
thermodynamic properties of mianserin suppositories have been
reported [13], and the pharmacokinetics of mianserin have been
compared between oral tablets and suppositories by Nawata et al.
[14]. However, the effectiveness in clinical cases has not yet been fully
compared between the oral tablet and suppository forms of mianserin;
also, the dose conversion ratio of these 2 forms remains unclear. This
study therefore investigated the effect of mianserin suppositories
in the treatment of delirium in cancer patients, in comparison with
therapeutic effect of mianserinoral tablets.
Figure 1
Table 1
Methods
Subjects
We retrospectively examined 66 cancers in patients who started
mianserin therapy with either oral tablets or suppositories to treat
delirium between April 1, 2013 and March 31 2015 at our cancer
center. All of the subjects who received suppositories had no options
for oral administration of mianserin and other medications.
Definition of patients’ background factors associated with
alleviation of delirium.
The following background factors of the patients influencing the
onset of delirium were examined and compared between 2 treatment
groups: daily dose of mianserin (either the oral tablet or suppository
form); age; sex; symptoms; the presence of brain tumors and/or brain
metastasis; the presence of Central Nervous System (CNS) disorders;
the presence of dementia; the presence of renal insufficiency; the
presence of hepatic insufficiency. Patients with CNS disorders were
defined as those with the history of dementia, Alzheimer’s disease,
and cerebrovascular disorder. In accordance with CTCAE (Common
Terminology Criteria for Adverse Events) ver.4.0, Grade ≥1 for blood
creatinine elevation (≥1.04 mg/dl in men, ≥0.79 mg/dl in women)
was defined as renal insufficiency, and Grade ≥1 for blood AST or
ALT elevation (an AST level ≥40 U/L or an ALT level ≥40 U/L) as
hepatic insufficiency. The Student’s t test was used for dose and age
comparisons, while the Fisher’s exact test was used for comparisons
of the other background factors of the patients.
Comparison of the response rate to therapy
Delirium was diagnosed based on the retrospective examination
of medical records in accordance with the Intensive Care Delirium
Screening Checklist (ICDSC) [15]. Responders to therapy were defined
as those who showed decreases in the ICDSC score (maximum score
of 8) from a pre-treatment score ≥4 to a post-treatment score ≤3 [16].
The first author of this study (R.T) was solely responsible for ICDSC
scoring. Response rates to mianserin by the 7th day of treatment were
compared between the oral tablet group and the suppository group
using Fisher’s exact test.
Comparison of adverse event incidence rates
Incidence rates of adverse events (hypersomnia, constipation, dry
mouth, urinary retention and nausea) at Grade 3 or higher according
to the CTCAE ver. 4.0, observed during a 1-week mianserin treatment
period were compared between the suppository group and the oral
tablet group using Fisher’s exact test.
Comparison of use of concomitant agents
Concomitant use of delirium-affecting agents, such as
psychotropic agents, opioids, benzodiazepines, steroids, H2 receptor
antagonists, and antihistaminergics [17], in a 2-week period (1
week before and 1 week after the start of mianserin therapy) were
compared between the oral tablet group and the suppository group
using Fisher’s exact test.
Ethical issues
This study adhered to the “Ethical Guidelines for Medical and
Health Research Involving Humans” and conducted after approval
by the institutional ethics committee (approval number 27-J154-27-
1-3).
Table 2
Table 3
Results
Among 66 subjects, 31 were in the suppository group, while
35 were in the oral tablet group. The daily doses of mianserin were
30-60 mg (median, 30 mg) and 10-60 mg (median 20 mg) in the
suppository group and in the oral tablet group, respectively (Table
1). There were no significant differences in the following between the
groups: age, sex, symptoms, and the presence of brain tumors/brain
metastasis, CNS disorders, dementia, past history of alcohol intake,
renal insufficiency, and hepatic insufficiency.
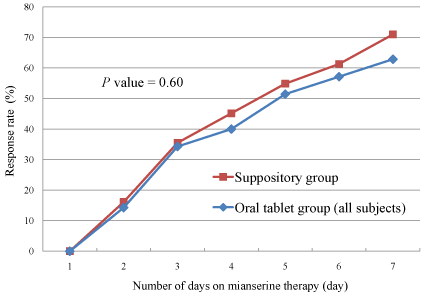
Overall response rates were 71.0% and 62.9% in the suppository
and in the oral tablet groups, respectively (Table 2), but the difference
was not statistically significant (Figure 2, P=0.60). The lowest possible
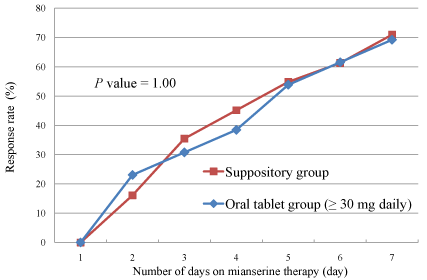
daily dose in the suppository group was 30 mg due to the formulation,
but a daily dose of 10-20 mg was administered in some patients in
the oral tablet group. The response rate in the oral tablet subgroup
receiving a daily dose ≥30 mg was 69.2%, which was very close to that
in the suppository group (Figure 3, P=1.00).
Regarding a comparison of adverse event incidence rates, there
were no significant differences in hypersomnia, constipation, dry
mouth, urinary retention, and nausea between the groups (Table 2).
Use of the following concomitant agents were examined:
psychotropic agents (haloperidol, chlorpromazine, levomepromazine,
risperidone, quetiapine, and mirtazapine); opioids (morphine,
oxycodone and tramal); benzodiazepines (etizolam, brotizolam,
flunitrazepam, alprazolam, lorazepam and diazepam), steroids
(prednisolone, hydrocortisone, dexamethasone and betamethasone),
H2 receptor antagonists (famotidine, ranitidine and lafutidine),
and antihistaminergics (chlorpheniramine, promethazine and
hydroxyzine). Use of the above agents with mianserin was not
significantly different between the groups (Table 3). Also, none of the
subjects consumed an over-the-counter product, health-enhancing
food, or an herbal product during the observation period.
Figure 2
Figure 3
Discussion
This study showed that the response rate to mianserin in
the suppository and the oral tablet forms were 71.0% and 62.9%
respectively, which were not significantly different. These rates were
consistent with the response rate in a previous study examining
delirium in Japanese patients (67.4%) [18], indicating that both the
suppository and oral tablet forms of mianserin exert a satisfactory
therapeutic effect in cancer patients with delirium. Mianserin
suppositories were offered selectively when the oral route was
unavailable, suggesting that mianserin suppositories will benefit
patients who are unable to take oral medications. It was also suggested
that a daily dose of 10-60 mg is appropriate clinically in cases of
delirium. However, in this study, the starting dose in each patient was
determined by the attending physician, and this may affect therapy
outcomes.
The results of the subgroup analysis comparing the 2 formulations
of mianserin in the matching dosing range showed that the
conversion ratio of an oral tablet to a suppository is probably 1:1. The
pharmacokinetics of mianserin suppositories showed a longer Tmax
and a lower AUC than that of the corresponding dose of mianserin
oral tablets [14]. However, this study did not find any significant
differences in therapeutic effect and the incidence rates of adverse
events in clinical cases.
Although many of the patients in this study used sleep-inducing
agents (e.g., psychotropic agents, opioids, and benzodiazepines)
in combination with mianserin, the incidence of hypersomnia
was similar to that previously reported for mianserin oral tablets
(23.4%) [10]. Also, some of the subjects received a psychotropic
agent (haloperidol, chlorpromazine, levomepromazine, risperidone,
quetiapine, and mirtazapine) for treatment of delirium. Although
the rates of use of concomitant agents were not significantly different
between the 2 groups, confounding factors (doses and frequencies of
administration) might have existed.
Furthermore, because this was a retrospective study, some
background factors that might have influenced the study results,
such as the presence of complications (e.g., abnormal levels of
blood electrolytes (e.g., Na+, K+, and Ca2+), hypoxemia, dehydration,
nutritional deficiency and endocrine disorders), were missing in
some patients, and thus were not examined.
Taken together, mianserin suppositories are comparable to
mianserinoral tablets in the treatment of delirium, and can bein
valuable for cancer patients when the oral route is unavailable.
References
- Lipowski ZJ. Delirium: Acute Confusional States. Oxford University Press. New York. 1990; 109.
- McNicol E, Horowicz-Mehler N, Fisk RA, Bennet K, Gialelo-Goudas M, Chew PW, et al. Management of opioid side effects in cancer related and chronic non-cancer pain: A systematic review. J Pain. 2003; 4: 231-256.
- Breitbart W, Marotta T, Platt MM, Weisman H, Derevenco M, Grau C, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996; 153: 231-237.
- Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007.
- Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs. lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007; 298: 2644-2653.
- Uchiyama M, Tanaka K, Isse K, Toru M. Efficacy of mianserin on symptoms of delirium in the aged: an open trial study. Prog Neuropsychopharmacol Biol Psychiatry. 1996; 20: 651-656.
- Uchimura N, Ohshima H. Alcohol withdrawal delirium. Japanese Journal of Clinical Psychopharmacology. 1998; 1: 1267-1275.
- Itoh H, Harada D, Hayashida K, Ishino Y, Nakayama K. Psychiatric medicine and sleeping disorders, delirium. Psychiatriaet Neurologia Japonica. 2006: 108; 1217-1221.
- Nakamura J, Uchimura N. Mianserin in treatment of delirium and its mechanism. Journal of Clinical and Experimental Medicine. 1995: 13; 1024-1025.
- Tetramide® tablets. Pharmaceutical interview form. MSD KK, the 11th edition; 2014.
- Serenace® tablets. Pharmaceutical interview form. Sumitomo Dainippon Pharma K K, the 22nd edition, 2014.
- Candy B, Jackson KC, Jones L, Leurent B, Tookman A, King M. Drug therapy for delirium in terminally ill adult patients. Cochrane Database Syst Rev. 2012: CD004770.
- Nakajima T, Iwata M, Nawata S, Saitoh H, Nakamura Y, Kobayashi Y, et al. Physicopharmaceutical characteristics of mianserin suppositories for inclusion in the hospital formulary. Pharmaceutical Health Care and Sciences. 2012: 38; 702-707.
- Nawata S, Kohyama N, Uchida N, Numazawa S, Ohbayashi M, Kobayashi Y, et al. The pharmacokinetics of mianserin suppositories for rectal administration in dogs and healthy volunteers: a pilot study. J Pharm Health Care Sci.2016; 2: 12.
- Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001; 27: 859-864.
- Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after onpump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012; 116: 987-997.
- Tuma R, DeAngelis LM. Altered mental status in patients with cancer. Arch Neurol. 2000; 57: 1727-1731.
- Nakamura J, Uchimura N, Yamada S. Therapeutic effect of mianserin in delirium, comparisons with oxyperitine and haloperidol. Japanese Journal of Neuropsychopharmacology. 1994: 14; 269-277.