Review Article
Relevance of Repeat Transurethral Resection of the Bladder for High Grade pTa Bladder Cancer: A Review
Priscilla Leon1, Adnan EL Bakri1, Fan Cheng2 and Stéphane Larré1,3*
1Department of Urology, Reims University, France
2Department of Urology, Renmin Hospital of Wuhan University, China
3Department of Urology, Tenon Hospital, France
*Corresponding author: Stéphane Larré, Department of Urology, CHU de Reims, Rue Cognacq Jay, 51 100 Reims, France
Published: 18 Oct, 2016
Cite this article as: Leon P, EL Bakri A, Cheng F, Larré S.
Relevance of Repeat Transurethral
Resection of the Bladder for High
Grade pTa Bladder Cancer: A Review.
Clin Oncol. 2016; 1: 1115.
Abstract
Repeat transurethral resection of the bladder (reTUR) is highly recommended for high risk non
muscle invasive bladder cancer. The evidence are strong for high grade pT1 tumors, but scarce for
high grade pTa. In this systematic pubmed review, we analyzed the performance of reTUR for high
grade pTa bladder cancer in terms of rate and characteristics of residual tumors. Data on survival
was also retrieved and we provided meta-analysis figures on what to expect with such approach.
ReTUR retrieved residual tumors in about half of the cases with upstaging to muscle invasive disease
in almost 2%. Data were nonetheless insufficient to state on the relevance of reTUR high grade pTa.
Based on analyzed data, we suggest to offer systematic reTUR for incomplete or muscle lacking
initial TUR. For other high grade pTa tumors, a second look TUR shall be an option to be discussed
at MDT meeting, based on the use of HAL or NBI at first TUR, the experience of the surgeon, and
whether the patient would be fit for a close surveillance or additional treatments.
Keywords: Urinary bladder neoplasms; Second-look surgery; Neoplasm; Residual; Repeat
resection; Transurethral resection of the bladder
Introduction
Non Muscle Invasive Bladder Cancer (NMIBC) are initially best managed using transurethral
resection of the bladder (TUR) aiming to provide adequate staging and tumor removal [1]. Although
TUR is widely used among urologists its efficacy is limited. Disease persistence or understating of
High Grade (HG) pT1 tumors has been reported as high as 72% and 29% respectively [2]. TUR
should therefore be seen as an incomplete procedure in most cases that requires additional measures
to improve the chance of cure.
Repeat transurethral resection of the bladder (reTUR) performed a few weeks after the initial
TUR has clearly proven to reduce the risk of recurrence for pT1 high risk NMIBC [3]. It improves
local control and response to BCG [4] and identifies a significant number of patient with muscle
invasive bladder cancer for which more invasive treatments are required. As a result, reTUR has
become the cornerstone of high risk NMIBC management and is strongly recommended by urology
guidelines [1,5].
The NMIBC high risk group also includes high grade pTa, and reTUR is recommended all
the same for this group although literature evidence is scarce to validate such approach. There is
even some controversy on whether reTUR should be offered for HG pTa cancer since the risk of
progression in this subgroup is much lower than for the pT1 group [6]. It is also unknown whether
early reTUR improves survival or quality of life compared to delayed TUR at the time of a recurrence
that would be spotted during surveillance.
We therefore aimed to review published literature to analyze the impact of reTUR for HG pTa
bladder cancer in terms of residual tumor yields, stages, morbidity, cost and survival and provide
overall meta-analysis figures on what to expect with such approach.
Methods
A Prisma systematic review of the literature was performed in august 2016 using Pubmed
with the following criteria:(“Urinary Bladder Neoplasms"[Mesh] OR “bladder cancer”) AND
("re-resection" OR "second look" OR "second TURB" OR "second resection" OR "persistent disease"
OR "Neoplasm, Residual"[Mesh] OR “re-staging” OR “restaging”
OR “repeat transurethral resection” OR “repeated transurethral
resection” OR “relook” OR "second-look surgery"[MeSH Terms]) AND
English[lang] NOT (Editorial[ptyp] OR Review[ptyp] OR Letter[ptyp]
OR Comment[sb] OR News[ptyp]).
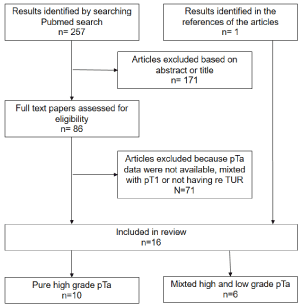
We retrieved 257 articles in July 2016. Further selection of
relevant articles was based on title, abstract and finally full paper
content analysis. Studies not including HG pTa tumors or studies
providing results mixing pTa and pT1 tumor results were discarded.
Any study providing either the rate of residual tumor or recurrence
rates for HG pTa were included. Studies in which HG and LG pTa
reTUR results were provided but mixed together were also selected
but analyzed separately. Further analysis of AUA, NICE and EAU
guidelines references and a similar criteria search focusing on reviews
only (n=63) was performed and retrieved one more article [7]. We
finally identified 16 articles among which 10 had detailed results for
HG pTa [7-15] and 6 with detailed results on a mixed group of low
and high grade pTa [16-21].
The stage of residual tumors was reported as a percentage of all
patients who had residual tumor on reTUR and for which the stage at
reTUR were reported. This explains why the sum of percentage in the
summaries is not 100%. Data on pT is on reTUR were merged with
pTa tumors in most of the publications and provided in the same way
for this review.
Table 1
Table 1
Summary of all studies reporting reTUR results for high grade or mixed grades pTa bladder cancers.
Single figures represent the number of patients. Figures in bold relates to the pTa category analyzed.
N/A: Not Analyzable; HG: High Grade Tumors or G2G3 tumors
*the first percentage relates to residual tumors only and the second to the total of patient who had reTUR.a
Results
Risk of residual disease on reTUR for HGpTa
Results are summarized in Table 1. Series including detailed data
for HG pTa reported the presence of residual disease on reTUR for
58% of the cases, ranging from 28% to 68%. In series for which LG
and HG pTa were analyzed together, the residual tumor rate was
lower (34% [27-52%]). This could possibly reflect the poor relevance
of reTUR for Low Grade (LG) pTa although the rate of HG pTa for
each series was unknown except for one [17]. In this study, authors
reported 82% of HG pTa and a residual tumor rate of 52% for all pTa
mixed together. As a matter of comparison, residual tumor rate on
reTUR for LG pTa was also provided in two studies of the review
[10,15] showing that among 49 and 40 LG pTa who had reTUR, 17
(35%) and 7 (18%) had residual tumors.
Many factors can influence residual tumor rates at reTUR [22],
especially the quality of the surgical technique used to perform the
initial TUR and its extension and depth. Surgeon’s technique and
experience is of importance since less recurrences were observed
for patients operated by senior urologist compared to residents
(Jancke, Rosell et al. 2014). Another related key factor is the presence
of detrusor on pathological reports confirming the initial TUR was
deep enough. This was not reported for pTa in most of the studies
included in this review [7,9,13,16-18,20,21,23]. Moreover, many did
not report whether all the patient included had a complete initial
TUR [7,9,12,13,17,21], and one series reported having included 12%
of incomplete initial TUR [16].
No studies reported on the performance of reTUR if the
initial TUR was followed by a single post-operative instillation
of mitomycin or performed using Narrow Band Imaging (NBI)
or hexylaminolevulinate acid (HAL). Although mitomycin in the
context of HG tumor is considered inapropriate, both NBI and HAL
have shown to reduce the risk of recurrence [1]. One randomized
study including HG pTa and pT1 compared white light reTUR to
NBI reTUR. All patients had white light TUR for the primary tumor.
Patients in the NBI reTUR had less recurrence at 2 years compared
to white light reTUR (22% vs. 33%), but the study was underpowered
and no data was available for HG pTa only. Similarly, another
randomized study compared HAL TUR to white light TUR followed
for both arms by white light cystoscopy [24]. HAL arm had less
recurrence and progression but no data could be extracted regarding
HG pTa.
Other factors may also influence residual tumor rates such as the
number of tumors resected and their size. These were poorly reported
for the pTa sub group. The tumor size was never reported and the
multifocality was only reported in two studies (14% [20] and 24%
[19]). Nonetheless, one study including only large (>3cm) or multiple
NMIBCa, reported the presence of residual macroscopic tumors at 3
months cystoscopy in 43.7% in a population of various grades pTa
that did not have reTUR [25]. This rate is comparable to the 51%
found for the mixed pTa population (Table 1).
Molecular factors may also influence the likelihood of residual
tumor on reTUR. Liu et al. [14], showed that tumor size, multifocality
but also p53 and E-cadherin expression are risk factors of residual
tumor at reTUR in a NMIBCa population. Such a marker panel may
help to identify patients with non-muscle-invasive bladder cancer
who are likely to have residual tumor after the first TUR and therefore
benefit from reTUR.
Finally, all series were retrospective and did not report clearly
on criteria triggering a reTUR nor did compare with pTa patients
that did not have a reTUR except two [7,8]. These limits have to be
taken into consideration and may result in significant differences in
reTUR tumor rates within centers and these results may not apply to
a population including all HG pTa.
Risk of upstaging on reTUR for HG pTa bladder cancer
ReTUR mainly serves one overarching purpose: to identify
patients with MIBCa who have been under staged by an inadequate
first TUR and would be inadequately treated without a reTUR.
When residual tumors were found on reTUR for HG pTa, a
similar stage was the most common occurrence. In the HG pTa series,
pTa stage represented 85% (71%-100%) of the residual tumors, and
73% (50%-83%) for mixed pTa series.
Regarding upstaging, although pT1 on reTUR was common
and occurred in 12% (0%-14%) for HGpTa and 25% (17%-50%) for
mixed pTa, upstaging to pT2 on reTUR was rare, occurring in 4%
(0%-13%) for both series.
It is remarkable that mixed series had a higher rate of upstaged
tumors than pure HG pTa series although they included some LG
pTa. As mentioned above, the presence of muscularis propria in
the sample was poorly reported by the authors although this is of
paramount importance to assess the technical quality of the TUR.
This may have had a role in the difference observed possibly explained
by more superficial TUR in the mixed pTa series. Nonetheless, it was
not possible to clarify this hypothesis in the studies.
The stages provided in these studies were also not reviewed
by multiple uro-pathologists. In a pathological review of samples
collected in 5 EORTC trials, agreement among pathologists was
low, reported to be only 57% for G1 pTa and even lower (50%) for
G3 pT1, [26]. The pathology review down staged 53% of the tumors
originally classified as pT1 to pTa. Such variations in the assessment
of the pathological stage may support the hypothesis that a significant
proportion of pTa that were upstages following reTUR may actually
be the result of initial TUR that were not deep enough or pT1 tumors
reported as pTa.
Nonetheless, upstaging to pT1 is unlikely to be a major issue
since management of HG pTa and pT1 bladder cancer follow similar
principles [1,5].
More of a concern is pT2 upstaging on reTUR. The risk of pT2
upstaging represented 4% of the residual tumors, but this was only 1.7
% of the 420 patients who had a reTUR (whatever the result) and for
which the data about pT2 upstaging was available. These results are
consistent with the recent EORTC nomogram for NMIBCa treated
with BCG and maintenance. None of the patient had reTUR in the
EORTC studies included. It showed that the 1 year progression rate
for HGpTa was 1.8% [6]. Since BCG is poorly effective when residual
tumor is left in place (especially for pT2 tumors), it can be hypothesize
that this early progression rate is the result of under diagnosed pT2
tumors. Of interest is also the progression rate for LG pTa in this
study which was very similar (1.9% and 1.6 % for G1 pTa and G2
pTa/G1 pT1 respectively). This suggest that the pT2 upstaging risk is
almost the same for HG pTa than LGpTa. Although pT2 upstaging is
of paramount importance since the management of muscle invasive
disease requires a much more invasive approach, it is questionable
to perform a systematic reTUR for all HG pTa (as suggested by
guidelines) since the pT2 upstaging risk may to be quite similar for
the whole pTa group.
What is the risk of recurrence and progression with or
without reTUR for HG pTa bladder cancer
Outcomes following reTUR were available in three studies that
compared reTUR and no reTUR arms for HGpTa tumors. Gendy et
al. [8] had a recurrence rate of 57% with reTUR compared to 83%
without, after a short follow-up of 3 to 6 months [13]. Although it
appears to be a strong difference between arms, the depth and
quality of the TUR were probably not optimal since only 36% of the
specimen had muscularis propria on pathological reports at initial
TUR. This would also explain that even in the reTUR arm, recurrence
rates were much higher than in another study from Vasdev et al. [7]
who reported recurrence rates of 13% with reTUR and 53% without
after 49 months. Differences in term of recurrence rate within studies
may have been explained by the quality of the initial TUR and the
administration of BCG, both in favor of the second study. In a third
study, authors reported less recurrences for patients not having had
reTUR (20% and 10% with and without reTUR) and both arms
received BCG [27]. Unfortunately no data were available regarding
residual tumor rates and stages in the reTUR arm.
All studies were retrospective, limited in size, without strict
criteria triggering the reTUR. The evidence are therefore insufficient
to draw any conclusion. Nonetheless, it is noteworthy that in these
studies, residual tumor rates were very close to recurrence rates in
the no reTUR arms(70% vs. 83% and 49% vs. 53% respectively),
suggesting that most of early recurrences are related to the presence
of residual tumor following the initial TUR.
Performing a reTUR in the pTa group is likely to reduce further
recurrence, but areTUR would be relevant only if it reduces the risk
of progression to muscle invasive disease or improves cancer specific
survival. None of the study reported on muscle invasive progression
during surveillance, but follow up was very limited for the first study
and concerned only 46 patients for the second. In this later, no cancer
specific deaths were recorded after 49 months.
Sfakianos et al. [28] reported updated survival outcome results of
a large series of 1021 high risk NMIBCa [28] including some selected
for this review published earlier [9]. Unfortunately, specific results
for the HGpTa that represented 60% of the cohort were not available.
Authors found that reTUR for high risk NMIBCa did not reduce the
risk of recurrence at 5 years when early recurrences were excluded
(within 3 months) compared to patients who did not have reTUR
(57.5% vs. 58.3% respectively).This study confirmed that there was
a reduction of the risk of progression for the entire cohort, but no
results were available for pTa tumors. This is likely to be biased by the
impact of reTUR on HGpT1 [3] and a potential benefit of reTUR on
HG pTa remains to be clarified.
Intravesical therapy is most effective when used against minimal
residual disease and is unlikely to eradicate macroscopic residual
tumors. Nonetheless, in a retrospective study including 56 newly
diagnosed G3pTa treated with BCG, post BCG recurrence occurred
for 2 out of in 10 patients who had reTUR (20%) compared to
10% for those 56 patients who did not have reTUR [27]. None had
progression. Authors concluded that a routine re-resection may
be unnecessary. Other authors have suggested that reTUR could
improve BCG response with the occurrence of lower grade tumors at
recurrence if reTUR did not harvest any tumor [11,14]. In this case,
reTUR would only be useful as a prognostic factor.
Overall, studies rarely analyze separately early and late recurrence
regarding the role of reTUR and its potential impact on intra-vesicle
therapies, and no conclusion could be drawn on the benefits of reTUR
on BCG response or progression for the HG pTa group.
Cost, morbidity and relevance of a reTUR
In terms of complications related to reTUR for HG pTa, no data
were available. In a study, only one out of 254 patients was returned
to the operating room for post-operative bleeding following reTUR
for high risk NMIBCa including 63% of HG pTa [29]. Another
large series reported that 2.8% of the patients will have bleeding
complications and 1.3% bladder perforation [30]. It is nonetheless
likely that reTUR may lead to less bleeding complications, since these
are related to the size and the number of tumors resected [30]. On the
contrary, one could suspect that reTUR may not lead to a lower rate
of bladder perforations. Less severe bladder injuries (0.3% vs. 0.6%)
were reported with the use of bipolar instead of monopolar TUR [31]
and may be used for patient undergoing reTUR.
No data were available regarding the cost of reTUR and we
found no medico economic studies on reTUR for HG pTa only. One
randomized study assessed HAL TUR for NMIBCa including 78% of
mixed grade pTa tumors in which all patients had reTUR [24]. Cost
analysis was in favor of performing HAL TUR followed by reTUR,
but no conclusion could be drawn for HG pTa.
Since the risk of misdiagnosing a muscle invasive tumor for HG
pTa is rare following the first TUR, it is questionable whether a reTUR
for all patients is relevant compared to performing a TUR when a
recurrence is observed following surveillance. Performing a reTUR
for all patients could be seen as an overtreatment since only half of the
tumors have residual tumors on reTUR, resulting in an increased cost
and increased risk of complications. It is also unknown whether the
almost 2% of patients found with muscle invasive disease at reTUR
would have been missed if reTUR would have been performed only
for incomplete initial TUR or for those lacking muscularis propria.
Moreover, HAL or NBI initial TUR although not available in all
centers is recommended and likely to minimize the relevance of
performing a systematic reTUR.
ReTUR is a generic procedure that encompass various situations
that should be define more precisely. New TUR performed because the
initial one was incomplete or lacking muscularis mucosae we suggest
it should be described as “complementary resection” or “restaging
resection” respectively. Excluding these two situations, when the TUR
is repeated because of the high risk of residual disease or under staging
based on stage or grade, we suggest it should be described as “secondlook
resection” to remain in line with the MeSH term “second-look
surgery”. In this review it was not possible to differentiate the type
of reTUR. Future studies on reTUR should differentiate thesevarious
situations. Moreover, this review highlighted the importance of early
versus late recurrence/progression and here again further studies may
benefit from analyzing this data separately.
Figure 1
Conclusion
ReTUR retrieved residual tumors in about half of the cases with upstaging to muscle invasive disease in almost 2%. Data were nonetheless insufficient to state on the relevance of reTUR for all HG pTa. Based on analyzed data, we suggest to offer systematic reTUR for incomplete or muscle lacking initial TUR. For other HG pTa tumors, a second look TUR shall be an option to be discussed at MDT meeting, based on the use of HAL or NBI at first TUR, the experience of the surgeon and whether the patient would be fit for a close surveillance or additional treatments. Further studies including all these aspects are required to clarify whether reTUR shall be offered routinely for all HG pTa.
References
- Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2016.
- Babjuk M. What is the optimal treatment strategy for T1 bladder tumors? Eur Urol. 2010; 57: 32-34.
- Divrik RT, Yildirim U, Zorlu F, Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol. 2006; 175: 1641-1644.
- Guevara A, Salomon L, Allory Y, Ploussard G, de la Taille A, Paul A, et al. The role of tumor-free status in repeat resection before intravesical bacillus Calmette-Guerin for high grade Ta, T1 and CIS bladder cancer. J Urol. 183: 2161-2164.
- Tan WS, Rodney S, Lamb B, Feneley M, Kelly J. Management of non-muscle invasive bladder cancer: A comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer treatment reviews. 2016; 47: 22-31.
- Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. European urology. 2016; 69: 60-69.
- Vasdev N, Mckie C, Dominguez-Escrig J, El-sherif A, Johnson M, Durkan GC, et al. The role of early re-resection in pTaG3 transitional cell carcinoma of the urinary bladder. British Journal of Medical and Surgical Urology. 2011; 4: 158-165.
- Gendy R, Delprado W, Brenner P, Brooks A, Coombes G, Cozzi P, et al. Repeat transurethral resection for non-muscle-invasive bladder cancer: a contemporary series. BJU Int. 2016; 117 4: 54-59.
- Herr HW, Donat SM. A re-staging transurethral resection predicts early progression of superficial bladder cancer. BJU Int. 2006; 97: 1194-1198.
- Cao M, Yang G, Pan J, Sun J, Chen Q, Chen Y, et al. Repeated transurethral resection for non-muscle invasive bladder cancer. Int J Clin Exp Med. 2015; 8: 1416-1419.
- Guevara A, Salomon L, Allory Y, Ploussard G, de la Taille A, Paul A, et al. The role of tumor-free status in repeat resection before intravesical bacillus Calmette-Guerin for high grade Ta, T1 and CIS bladder cancer. J Urol. 2010; 183: 2161-2164.
- Han CH, Shi Z, Xuan X, Chen B, Dong B, Hao L, et al. Re-transurethral resection treatment for non-invasive bladder tumor. Cell Biochem Biophys. 2014; 69: 589-592.
- Lazica DA, Roth S, Brandt AS, Bottcher S, Mathers MJ, Ubrig B. Second transurethral resection after Ta high-grade bladder tumor: a 4.5-year period at a single university center. Urol Int. 2014; 92: 131-135.
- Liu W, Qi L, Zu X, Li Y, He W, Tong S, et al. A preoperative marker panel for the prediction of residual tumor and the decision making for repeat transurethral resection. Urol Oncol. 2015; 33: 9-14.
- Fujikawa A, Yumura Y, Yao M, Tsuchiya F, Iwasaki A, Moriyama M. An evaluation to define the role of repeat transurethral resection in a treatment algorithm for non-muscle-invasive bladder cancer. Indian J Urol. 2012; 28: 267-270.
- Ali MH, Ismail IY, Eltobgy A, Gobeish A. Evaluation of second-look transurethral resection in restaging of patients with nonmuscle-invasive bladder cancer. J Endourol. 2010; 24: 2047-2050.
- Bishr M, Lattouf JB, Latour M, Saad F. Tumour stage on re-staging transurethral resection predicts recurrence and progression-free survival of patients with high-risk non-muscle invasive bladder cancer. Can Urol Assoc J. 2014; 8: 306-310.
- Gill TS, Das RK, Basu S, Dey RK, Mitra S. Predictive factors for residual tumor and tumor upstaging on relook transurethral resection of bladder tumor in non-muscle invasive bladder cancer. Urol Ann. 2014; 6: 305-308.
- Grimm MO, Steinhoff C, Simon X, Spiegelhalder P, Ackermann R, Vogeli TA. Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study. J Urol. 2003; 170: 433-437.
- Schips L, Augustin H, Zigeuner RE, Galle G, Habermann H, Trummer H, et al. Is repeated transurethral resection justified in patients with newly diagnosed superficial bladder cancer? Urology. 2002; 59: 220-223.
- Zurkirchen MA, Sulser T, Gaspert A, Hauri D. Second transurethral resection of superficial transitional cell carcinoma of the bladder: a must even for experienced urologists. Urol Int. 2004; 72: 99-102.
- Brausi M, Collette L, Kurth K, van der Meijden AP, Oosterlinck W, Witjes JA, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002; 41: 523-531.
- Richterstetter M, Wullich B, Amann K, Haeberle L, Engehausen DG, Goebell PJ, et al. The value of extended transurethral resection of bladder tumour (TURBT) in the treatment of bladder cancer. BJU Int. 2012; 110: 76-79.
- Daniltchenko DI, Riedl CR, Sachs MD, Koenig F, Daha KL, Pflueger H, et al. Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. J Urol. 2005; 174: 2129-2133.
- Un S, Turk H, Karabicak M, Divrik RT, Zorlu F. Is a second look necessary in multiple and/or large Ta tumors? Arch Ital Urol Androl. 2016; 88: 86-88.
- Van Der Meijden A, Sylvester R, Collette L, Bono A, Ten Kate F. The role and impact of pathology review on stage and grade assessment of stages Ta and T1 bladder tumors: a combined analysis of 5 European Organization for Research and Treatment of Cancer Trials. J Urol. 2000; 164: 1533-1537.
- Holmang S. High-grade non-muscle-invasive bladder cancer: is re-resection necessary in all patients before intravesical bacillus Calmette-Guerin treatment? Scand J Urol. 2013; 47: 363-369.
- Sfakianos JP, Kim PH, Hakimi AA, Herr HW. The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette-Guerin. J Urol. 2014; 91: 341-345.
- Herr HW. Randomized trial of narrow-band versus white-light cystoscopy for restaging (second-look) transurethral resection of bladder tumors. Eur Urol. 2015; 67: 605-608.
- Collado A, Chechile GE, Salvador J, Vicente J. Early complications of endoscopic treatment for superficial bladder tumors. J Urol. 2000; 164:1529-1532.
- Sugihara T, Yasunaga H, Horiguchi H, Matsui H, Nishimatsu H, Nakagawa T, et al. Comparison of perioperative outcomes including severe bladder injury between monopolar and bipolar transurethral resection of bladder tumors: a population based comparison. The Journal of urology. 2014; 192: 1355-1359.