Short Review/Commentary
PIK3CA Gene Mutations in Major Gynecologic Cancers
Akram Husain RS and Ramakrishnan V*
Genetics Lab, Faculty of Allied Health Sciences, Chettinad Academy of Research and Education, India
*Corresponding author: Ramakrishnan V, Faculty of Allied Health Sciences, Chettinad Academy of Research and Education, Chettinad Health City, Kelambakkam-603 103, Tamil Nadu, India
Published: 28 Sep, 2016
Cite this article as: Akram Husain RS, Ramakrishnan V. PIK3CA Gene Mutations in Major Gynecologic Cancers. Clin Oncol. 2016; 1: 1110.
Short Review/Commentary
Gynecologic malignancies posses’ major threat in women, leading to cancer in their
reproductive organs, there are five main types including cervical, ovarian, uterine, vaginal and
vulvar [1]. Traditionally, cancers have been viewed as diseases that are caused by the accumulation of genetic mutations, epigenetic alterations and environmental risk factors [2]. The development of sequencing era lead to identification of candidate genes, oncogenes, tumor suppressor genes which
are directly associated with the disease [3]. These gynecological cancers account for 5.1 million new cancer cases among women every year globally. Out of these five malignancies cervical and ovarian
cancers are more common affecting women from 30 years and above with high incidence rates [4].
In brief, cervical cancer defined as the malignant neoplasm arising from cells originating in the
cervix and the cancer in any one of the ovaries are termed as ovarian cancer. Comparative Genomic
Hybridization (CGH) studies have documented the amplification of 3q region in various tumor
types, which found PIK3CA at 3q26 encoding the p110a catalytic subunit of phosphatidylinositol
(PI) 3-kinase as an oncogene in cervical [5] and ovarian cancer [6]. This review focuses on the reported genetic mutations occurring in Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA) oncogene of cervical and ovarian cancer respectively.
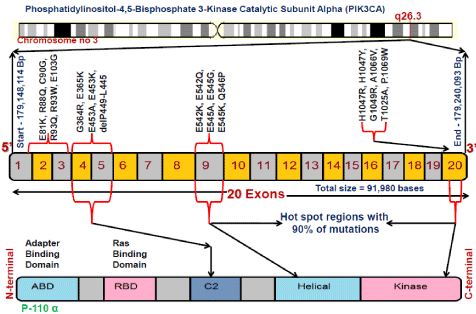
PIK3CA gene spanning 20 exons encodes for protein of 1068 amino acids, 9th and 20th exons
are considered as mutational hot spots harboring more somatic mutations playing significant roles
in various stages of cancers [7]. (Figure1) explains the chromosomal locations, exon structure, location of reported mutations at their corresponding genomic positions and the protein domains. The p110α subunit consists of five domains namely: ABD (adaptor-binding domain, RBD (Rasbinding domain), C2 domain, helical and kinase domains. The PI3K-AKT signaling pathway mainly involve in the regulation of various biological processes including cell propagation, progression and survival [8]. Abnormal activation of this pathway has been observed in various types of gynecologic cancers, causing aberrant cell-cycle progression, altered adhesion, apoptosis inhibition, motility and angiogenesis indicating the significance of PI3K pathway in carcinogenesis [9].
In cervical cancer, recent study in 2015 published from Chinese population with 771 cervical
cancer patients screened for PIK3CA gene documented 13.6% subject’s harbored non-synonymous
mutations from the 9th and 20th exons in which most of them are found to be pathogenic [10]. A study comprising 114 cervical cancer samples from Italy revealed 11%
of mutations in this gene in different stages of cervical malignancy
[11]. Similar study with 255 cancer patients from Sweden and China
were analyzed exon 1, 9, 20 of PIK3CA gene in cervical carcinomas
identified 8.15% of somatic mutations suggesting that genetic
alterations of this oncogene are late events during the process of
carcinogenesis [12]. The functional consequence of these mutations
leads to over expression of mutant PIK3CA proteins causing the
cellular transformation with phosphorylation of proteins in the AKT
signaling pathway [13].
The effects of PIK3CA gene mutations in ovarian cancer were
same as the cervical malignancies with higher percentage of variations
in exon 9, 20 comprising the helical and kinase domains [14]. A study
published in 2005 with 198 cancer patients from USA revealed 12%
of mutations signifying the oncogenic role of PIK3CA gene and
its pathway in ovarian cancers [15]. Similar study, comprising 182
ovarian cancer subjects from Australia documented with mutational
frequency of 6%, these mutations played major role in pathogenesis
of this disease. The presence of hotspot regions strongly elucidate
that mutant protein may be associated with higher kinase activity
and oncogenic properties [16]. In-spite of several confounding
factors such as sample source, collection, preservation methods,
geographical variations, ethnic background, DNA isolation, PCR
methods also have an impact on mutation detection. Despite these
limitations, the high frequencies of PIK3CA gene mutations have
an impact on clinical applications for cancer diagnosis, prognosis of
disease followed by therapeutic measures.
Figure 1
References
- Mishra K. Gynaecological malignancies from palliative care perspective. Indian journal of palliative care. 2011; 17: 45-50.
- You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer cell. 2012; 22: 9-20.
- Osborne C, Wilson P, Tripathy D. Oncogenes and tumor suppressor genes in breast cancer: potential diagnostic and therapeutic applications. The oncologist. 2004; 9: 361-377.
- Cui B, Zheng B, Zhang X, Stendahl U, Andersson S, Wallin KL. Mutation of PIK3CA: possible risk factor for cervical carcinogenesis in older women. Int J oncol. 2009; 34: 409-420.
- Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC, Whang-Peng J, et al. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000; 19: 2739-2744.
- Lockwood WW, Coe BP, Williams AC, MacAulay C, Lam WL. Whole genome tiling path array CGH analysis of segmental copy number alterations in cervical cancer cell lines. Int J Cancer. 2007; 120: 436-443.
- Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014; 506: 371-375.
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002; 296: 1655-1657.
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nature Reviews Cancer. 2002; 2: 489-501.
- Xiang L, Jiang W, Li J, Shen X, Yang W, Yang G, et al. PIK3CA mutation analysis in Chinese patients with surgically resected cervical cancer. Sci Rep. 2015; 5: 14035.
- Tornesello ML, Annunziata C, Buonaguro L, Losito S, Greggi S, Buonaguro FM. TP53 and PIK3CA gene mutations in adenocarcinoma, squamous cell carcinoma and high-grade intraepithelial neoplasia of the cervix. J Trans Med. 2014; 12: 225.
- Bertelsen BI, Steine SJ, Sandvei R, Molven A, Laerum OD. Molecular analysis of the PI3K‐AKT pathway in uterine cervical neoplasia: Frequent PIK3CA amplification and AKT phosphorylation. Int J Cancer. 2006; 118: 1877-1883.
- Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006; 94: 455-459.
- Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer research. 2004; 64: 7678-7681.
- Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clinical Cancer Research. 2005; 11: 2875-2878.
- Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res. 2014; 16: 201.