Case Report
Isolated Drain Site Metastasis after Laparoscopic Radica Pancreatosplenectomy in Left-Sided Pancreatic Cancer
Sung Whan Cha, Chang Moo Kang* and Woo Jung Lee
Department of Surgery, Yonsei University College of Medicine, Korea
*Corresponding author: Chang Moo Kang, Department of Surgery, Yonsei University College of Medicine, Severance Hospital, 50 Yonsei-Ro, Seodaemun-Ku, Seoul, 03722, Korea
Published: 28 Sep, 2016
Cite this article as: Cha SW, Kang CM, Lee WJ. Isolated Drain Site Metastasis after Laparoscopic Radical Pancreatosplenectomy in Left-Sided Pancreatic Cancer. Clin Oncol. 2016; 1: 1106.
Abstract
In spite of debates, minimally invasive radical distal pancreatosplenectomy is carefully thought to be safe and effective in well selected left-sided pancreatic cancer. Several significant literatures are
demonstrating that it can result in comparable oncologic outcomes of conventional open distal pancreatectomy. It is general that patients with resected pancreatic cancer experience systemic metastasis; however, cutaneous metastasis of resected pancreatic cancer is reported to be rare. Here, we report extremely rare case of isolated drain-site metastasis of resected left-sided pancreatic cancer.
Keywords: Minimally invasive surgery; Laparoscopic; Pancreatectomy; Pancreatic cancer; Metastasis
Introduction
With the advance of minimally invasive surgery, laparoscopic distal pancreatectomy is regarded as safe and effective approach in treating non-malignant pancreatic pathology. Although it is
controversial, laparoscopic or robotic distal pancreatosplenectomy is carefully reported to be one of
the options for curative surgery for well-selected left-sided pancreatic cancer [1,2].
It is general that pancreatic cancer is famous for early recurrence even after radical
pancreatectomy. Liver is the most common site of recurrence, followed by lungs, bones, and brain
[3,4]. So called, Sister Mary Joseph’s nodule is known for one of the predominant presentations of cutaneous metastasis. Non-umblical cutaneous metastasis of pancreatic cancer is very rare. Most
cases are usually associated with disseminated pancreatic cancer [5].
In this study, we present extremely rare case of isolated drain-site metastasis of resected leftsided
pancreatic cancer. This report is thought to be the first to show the potential risk of drain-site
recurrence after laparoscopic radical distal pancreatosplenectomy, providing new insight for careful
follow-up of pancreatic cancer patients who underwent minimally invasive radical pancreatectomy.
Case Presentation
A fifty-one year old female patient visited our hospital for palpable mass on left mid-abdominal
area. She had undergone laparoscopic radical pancreatosplenectomy for left sided pancreatic cancer
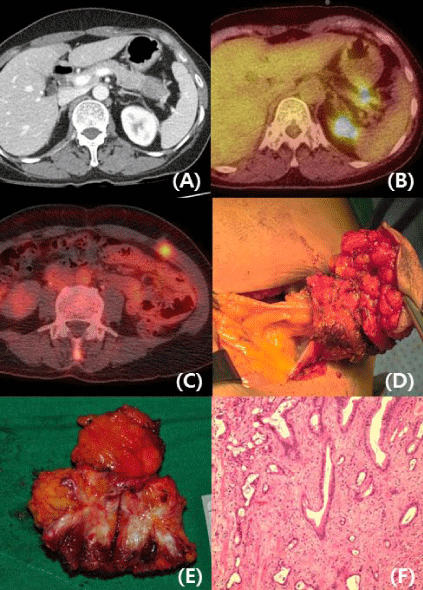
on 18 August, 2015 (Figure 1A and B). During initial preoperative evaluation period for primary
left-sided pancreatic cancer, a gastroenterologist had attempted endoscopic ultrasound-guided
fine needle biopsy (EUS-FNAB), but failed to confirm pathologic diagnosis. Preoperative CA 19-9
was noted to be 223.5 U/ml. Minimally invasive radical distal pancreatosplenectomy was done by
previously reported approach [1]. After surgery, no clinically relevant postoperative complication was
noted, and could go home on postoperative seventh day. She started chemotherapy (Gemcitabine)
on postoperative twenty eighth days, and had completely the systemic treatment. Follow up the
serum CA 19-9 had dropped down to 11.6U.
On postoperative 8 months, she reported painless abdominal wall lump around previous drain
site. It was noted that serum CA 19-9 elevated up to 40.0 U/ml and PET-CT showed increased signal
intensity on left-mid abdominal wall area compatible with previous drain insertion site following
initial laparoscopic radical pancreatectomy (Figure 1C). There are no other evidences suggesting
associated systemic or peritoneal recurrence. Wide excision of isolated drain-site metastasis was
performed with approximately 1.5 cm margin (Figure 1D and E). Pathologic examination revealed
that about 2 cm x 1 cm x 1 cm sixed mass was metastatic adenocarcinoma, clinically from pancreas
(Figure 1F).
Figure 1
Discussion
Since 2007, under our own strict selection criteria, so called
Yonsei Criteria [6-8], we have applied minimally invasive radical
distal pancreatosplenectomy in left-sided pancreatic cancer. Our
institutional experiences of radical distal pancreatectomy for
pancreatic cancer suggested that bloodless and margin negative
resection was identified to be independent prognostic factors for
left-sided pancreatic cancer [9]. On preoperative CT scan, following
tumor characteristics will be appropriate for bloodless and marginnegative
minimally invasive distal pancreatectomy; 1) pancreasconfining
tumor, 2) intact fascia layer between left-sided pancreas
and left adrenal gland and kidney, and 3) tumor at least 1-2cm apart
from celiac axis (origin of the splenic artery) [10]. Recently, it was
reported that minimally invasive radical distal pancreatectomy for
pancreatic cancer within Yonsei Criteria resulted in very excellent
oncologic outcomes [1]. Although our Yonsei Criteria needs for
external validation, it seems to provide the insight for decisionmaking
in treating left-sided pancreatic cancer [11].
With the increasing clinical practices of minimally invasive radical
surgery in clinical oncology, skin metastases are not uncommonly
reported. Especially, skin metastasis from gallbladder cancer and
colon cancer after laparoscopic minimally invasive surgery were often
reported [12,13]. However, as far as authors’ knowledge, the present
case of isolated drain site metastases from pancreatic cancer following
laparoscopic radical distal pancreatectomy is the first report in far
advanced laparoscopic era.
When reviewing the literatures [5,14], cutaneous metastasis after
radical pancreatectomy for left-sided pancreatic cancer was frequent,
and almost all cases were noted to be associated with disseminated
disease. Although there were no evidences of multiple systemic
metastases, our patient needed for close observation and additional
systemic chemotherapy. In fact, the patient refused additional
systemic chemotherapy. During follow up, she was found to have multiple peritoneal seeding 4 months after excisional biopsy for
cutaneous lesion.
Regarding mechanisms of cutaneous metastasis after minimally
invasive radical pancreatectomy for left-sided pancreatic cancer,
several issues can be taken into consideration. First, EUS-FNAB
associated peritoneal resected pancreatic seeding is concerned.
Although it is known that preoperative EUS-FNAB does not impair
survival of patients with cancer [15], there are several reports on
needle tract, perigastric and peritoneal seeding after EUS-guided
fine needle aspiration biopsy in pancreatic cancer [16-18]. Second,
according to the pathological examination, cancer cells were found
to invade to peripancreatic soft tissue (pT3), which might be very
vulnerable for shedding during laparoscopic mobilization of resected
left-sided pancreas. Third, adverse impact of tumor biology itself
needs to be taken into consideration. For example, previous our study
correlating SUVmax determined based on preoperative PET-CT and
SMAD4 expression, high SUVmax value was found to be closely related
to the loss of SMAD4, suggesting systemic metastasis [19].
According to our experiences of minimally invasive approach to
well selected left sided pancreatic cancer, oncologic outcome was very
excellent, however, the present case is again giving a fundamental
lesson that pancreatic surgeon should be alert to isolated drain site
recurrence of the pancreatic cancer. Careful wound inspection and
physical examination will facilitate early detection of cutaneous
metastasis, which might be associated with disseminated tumor
conditions. In addition, it has been reported that FOLFIRINOX
and nab-paclitaxel are very effective in systemic pancreatic cancer
[20]. Additional aggressive chemotherapy may be only chance for
improving survival outcome for this patient.
References
- Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ, Chi HS. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc. 2014; 28: 2848-2855.
- Postlewait LM, Kooby DA. Laparoscopic distal pancreatectomy for adenocarcinoma: safe and reasonable?. J Gastrointest Oncol. 2015; 6: 406-417.
- Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26: v56-v68.
- Satoh K, T Shimosegawa. [Pancreatic tumor: progress in diagnosis and treatment. Topics: I. Pancreatic carcinoma: 2. Pathogenesis and pathobiology in pancreatic cancer. The molecular mechanisms of carcinogenesis, and invasion and metastasis in pancreatic cancer]. Nihon Naika Gakkai Zasshi. 2012; 101: 7-16.
- Zhou HY, Wang XB, Gao F, Bu B, Zhang S, Wang Z. Cutaneous metastasis from pancreatic cancer: A case report and systematic review of the literature. Oncol Lett. 2014; 8: 2654-2660.
- Choi SH, Kang CM, Hwang HK, Lee WJ, Chi HS. Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg. 2012; 16: 868-869.
- Choi SH, Kang CM, Lee WJ, Chi HS. Multimedia article. Laparoscopic modified anterior RAMPS in well-selected left-sided pancreatic cancer: technical feasibility and interim results. Surg Endosc. 2011; 25: 2360-2361.
- Han DH, Kang CM, Lee WJ, Chi HS. A five-year survivor without recurrence following robotic anterior radical antegrade modular pancreatosplenectomy for a well-selected left-sided pancreatic cancer. Yonsei Med J. 2014; 55: 276-279.
- Kang CM, DH Kim, WJ Lee. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc. 2010; 24: 1533-1541.
- Kang CM, SH Lee, WJ Lee. Minimally invasive radical pancreatectomy for left-sided pancreatic cancer: current status and future perspectives. World J Gastroenterol. 2014; 20: 2343-2351.
- de Rooij T, Klompmaker S, Abu Hilal M, Kendrick ML, Busch OR, Besselink MG. Laparoscopic pancreatic surgery for benign and malignant disease. Nat Rev Gastroenterol Hepatol. 2016; 13: 227-238.
- Bărbulescu M, Alecu L, Boeţi P, Popescu I. Port-site metastasis after laparoscopic surgery for colorectal cancer--still a real concern? Case report and review of the literature. Chirurgia (Bucur). 2012; 107: 103-107.
- Lupinacci RM, Santana A, Dias AR. Metastatic gallbladder adenosquamous carcinoma to the skin. J Surg Case Rep. 2014; 2014.
- Kaoutzanis C, Chang MC, Abdul Khalek FJ, Kreske E. Non-umbilical cutaneous metastasis of a pancreatic adenocarcinoma. BMJ Case Rep. 2013; 2013.
- Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015; 64: 1105-1110.
- Yamao K. Complications of endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB) for pancreatic lesions. J Gastroenterol. 2005; 40: 921-923.
- Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005; 61: 610-611.
- Chong A, Venugopal K, Segarajasingam D, Lisewski D. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011; 74: 933-935.
- Kang CM, Hwang HK, Park J, Kim C, Cho SK, Yun M, et al. Maximum Standard Uptake Value as a Clinical Biomarker for Detecting Loss of SMAD4 Expression and Early Systemic Tumor Recurrence in Resected Left-Sided Pancreatic Cancer. Medicine (Baltimore). 2016; 95: e3452.
- Rajeshkumar NV, Yabuuchi S, Pai SG, Tong Z, Hou S, Bateman S, et al. Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br J Cancer. 2016; 115: 442-453.