Research Article
Treatment-Related MR Imaging Findings in Patients with Glioma after Radiotherapy, Chemotherapy, and Biological Therapy
Xiao Li and Fanny Morón*
Department of Radiology, Baylor College of Medicine, USA
*Corresponding author: Fanny Morón, Department of Radiology, Baylor College of Medicine, Houston, TX, US, One Baylor Plaza, Houston, TX 77030, USA
Published: 28 Sep, 2016
Cite this article as: Li X, Morón F. Treatment-Related MR Imaging Findings in Patients with Glioma after Radiotherapy, Chemotherapy, and Biological Therapy. Clin Oncol. 2016; 1: 1103.
Abstract
Patients undergoing radiotherapy, chemotherapy, and biological therapy demonstrate treatmentrelated
changes and characteristic magnetic resonance imaging findings. By understanding the
various imaging findings, better clinical decisions can be made.
Glioblastoma is the most common malignant primary brain tumor. Current standard of care
involves surgical resection with first line radiation therapy and concomitant/adjuvant temozolomide
chemotherapy and second line anti-angiogenic therapy, bevacizumab, for recurrent glioblastoma.
Macdonald‘s criteria were commonly used for assessing treatment response to high-grade glioma
therapy. Limitations to these criteria led to the more updated response assessment in neurooncology
criteria.
Intracranial and regional radiation to the brain often produces a number of significant changes that
complicate the assessment of post-treatment outcomes. Leukoencephalopathy is often seen postradiation
therapy because white matter is particularly vulnerable to radiation. Radiation necrosis
and tumor reoccurrence can both present with contrast enhancement, mass effect and vasogenic
edema. Other post-radiation therapy changes include radiation-induced meningioma, cavernous
malformation, and micro bleeds.
Chemotherapy and biological therapy treatments also create difficulty in post-treatment response
interpretation. For example, pseudop rogression is non-tumoral enhancement likely associated with
inflammatory local tissue reaction and edema caused by treatments such as temozolomide. On the
other hand, pseudo response is post-treatment decrease in contrast enhancement not associated
with true tumor reduction.
Keywords: Abnormal regional wall motion; High sensitivity c-reactive Protein; Hematopoietic stem cell transplantation
Introduction
To provide a mini-review of the various treatment related changes and characteristic MR imaging findings in patients with glioma after undergoing radiotherapy, chemotherapy and biological therapy.
Materials and Methods
A literature review was performed to discuss the treatment changes and imaging appearances followed by a pictorial essay of selected examples.
Results and Discussion
Treatment for malignant neoplasm such as glioblastoma has been standardized with surgery,
radiation treatment and temozolomide. In 2009, Bevacizumab was approved for recurrent
glioblastoma. Historically, Macdonald’s criteria were used to assess treatment response based on
tumor size on Magnetic Resonance (MR), clinical assessment, and corticosteroid use [1]. However,
Macdonald’s criteria were limited because it only factored in the contrast-enhancing tumor
component and did not adequately deal with pseudo progression and pseudo response, discussed
later. Response Assessment in Neuro-oncology criteria (RANO) were created as an updated
guideline by taking into account the non-enhancing tumor component [2,3]. Patients undergoing
radiotherapy, chemotherapy, and biological therapy demonstrate the following treatment-related
changes and characteristic MR imaging findings.
Radiation-related treatment effects
Radiation therapy is typically administered 5 days a week for 6-7
weeks until a dose of 60 Gy is reached. The radiation target dividing
cells directly and indirectly leading to vasodilation, disruption of the
blood brain barrier, and edema [3]. This leads to vascular damage,
endocrine disturbance, and neural structural fibrosis [4].
There are multiple phases of radiation injury. Acute phase of
radiation injury occurs during or shortly after radiation with only
focal damage with reversible glial glycogen depositions. The sub acute
phase occurs up to 12 weeks after radiation with cell death of myelinproducing
oligodendrocytes that followsremyelinization of the brain
tissue. The chronic phase occurs months to years after completing
radiation with diffuse changes due to wall thickening of the vascular
structures, decreasing number of glial-supporting cells, and diffuse
demyelinization [1,3,5,6].
Enhancement on MR can also be caused by many factors from
tumor reoccurrence to inflammation (treatment-related), postsurgical
changes, ischemia, and radiation effects [3]. Post-surgical
enhancement possibly due to ischemic changes may occur shortly
after the procedure and can last sub acutely up to 2-3 months
[3]. Two major findings that can come about after radiation are
leukoencephalopathy and radiation necrosis.
Leukoencephalopathy often develops months to years after
treatment. This can be accompanied by chronic mental status
impairment with progressive cognitive decline and personality
changes [4]. It is often seen in post-radiation therapy and exacerbated
in combination with chemotherapy. MRI findings include progression
of white matter confluent FLAIR hyperintensity, progressive atrophy, and transient areas of white matter enhancement [7] (Figure1).
Ventricular dilation, cerebral atrophy, and areas of focal enhancement
may also be seen which can lead to weakness, dementia, and death [4].
Radiation necrosis has an incidence of 3-24% and often occurs
3-12 months after treatment, but can also present up to decades later
[8]. The radiation potentiates in combination with chemotherapy
to increase the risk. Overall, 70% of radiation necrosis is stable or
improved. 30% of radiation necrosis are variable on follow-up that
may reappear, progress (with re-radiation), or have new distant
lesions.
Radiation necrosis has particular patterns of enhancement. There
may be new enhancement on initially non-enhancing tumor, distant
enhancing foci, periventricular and callosal enhancing foci, and soap
bubble or Swiss cheese patterns (Figure 2). Periventricular white
matter is one of the most susceptible areas to radiation necrosis [6].
The soap bubble pattern results from diffuse necrosis affecting the
white matter and adjacent cortex while the Swiss cheese pattern is
typically more diffuse and larger in area [6,8].
It can be challenging to differentiate between radiation necrosis
versus reoccurrence of the tumor. Similarities include contrast
enhancement, mass effect, and vasogenic edema. However, tumor,
not radiation necrosis, has increased relative cerebral blood volume
(rCBV). Advanced imaging of radiation necrosis demonstrates
low rCBV, no restricted diffusion, and low choline peak on MR
spectroscopy. Radiation necrosis tends to be stable or improve over
time. Also, if enhancing lesions develop at a distance away from
the primary tumor, radiation necrosis should first be suspected
[6]. Additionally, combination of corpus callosum involvement and multiple enhancing lesions +/- crossing the midline and
subependymal spread favors tumor progression [9].
Radiation-induced meningioma is the most common central
nervous system neoplasm caused by ionizing radiation (Figure 3). Risk
increases with increased doses. Even low doses significantly increase
the risk of inducing meningioma. Higher proportions of multiple
meningiomas and atypical or anaplastic meningiomas are observed
in patients who have received radiation therapy compared to those
patients who have not. Patients who have received radiation have a
lower mean age of presentation, 29 to 38 years for those exposed to
high dose radiation compared to 45-58 years in spontaneous cases
[10].
Radiation can cause early vascular changes such as increased
capillary permeability, vasodilation and delayed injury leading to
occlusion/infarction, as well as proliferative changes such as capillary
telangiectasia and cavernous malformation. Capillary telangiectasias
are thin-walled capillaries with intervening normal brain parenchyma
and occur 3-9 months after irradiation. Cavernous malformations
do not contain the intervening brain parenchyma and tend to
develop years later. Cavernous malformation, on imaging, presents
with distinctive “popcorn” appearance with minimal surrounding
edema (Figure 4). On CT, there may be ring-like calcifications with
core reticulation of variable attenuation. On MR, there may be core
heterogeneous signal intensity with dark peripheral hemosiderin rim [11].
Radiation can cause cerebral hemorrhage resulting in the
formation of cerebral microbleeds. These microbleeds contain focal
perivascular collections of hemosiderin and persist for years [12].
Hemosiderin contains iron and has associated susceptibility effects.
MR may show small, round, hypointense lesions on T2*-weighted
images obtained using gradient echo or susceptibility-weighted
sequences (Figure 5). There is increase in number of lesions over
time after irradiation that correlates with dose and target volume.
Therefore, monitoring these lesions may be a useful measurement of
radiation injury [12].
Chemotherapy imaging-related changes
Traditional chemotherapy primarily induces DNA damage to
dividing cells and/or interferes with DNA repair [4]. The addition of
temozolomide (TMZ) chemotherapy to radiation therapy was shown
to increase mean survival in newly diagnosed glioblastoma from 12.1
to 14.6 months [8]. Following treatment with TMZ, a phenomenon
of increased contrast-enhancing lesion size was documented [8]
(Figure 6). This was later termedpseudoprogression and is defined by
non-tumoral increased enhancement of lesion after treatment seen
in approximately 20% of patients treated with concomitant TMZ
and radiotherapy [8]. Mechanism of pseudoprogression involves
demyelination secondary to hypoxia and endothelial damage,
necrosis, and activation of VEGF (due to increase permeability of blood-brain barrier leading to enhancement and vasogenic edema).
Chemotherapy treatments can create difficulty in post-treatment
response interpretation. By the Macdonald’s criteria, this enhancement
may be interpreted as tumor progression using traditional T1-
weighted post contrast scans. Hence, Response Assessment in Neurooncology
(RANO) criteria is an updated methodology for treatment
response. RANO uses FLAIR/T2 hyperintensity as a surrogate for
non-enhancing tumor. One drawback of RANO is that it can difficult
to differentiate between similar appearing FLAIR/T2 hyperintensity
such as that caused by radiation-induced gliosis [13]. Much of the
current research involves the search for physiologic rather than
anatomic techniques to assess tumor response.
MR is unable to directly differentiate between pseudoprogression
versus early disease progression except by evaluating changes on
follow-up exams [3]. Most cases of pseudoprogression result in
spontaneous “remission” in the first 3 months or stability for 6
months. Pseudoprogression is a sub acute change that can occur
with or without clinical deterioration, but most patients do not show
clinical symptoms despite increased radiologic abnormalities [3]. It is
shown that pseudoprogression development actually correlates with
better outcome and survival. This may be due to possible anti-tumor
inflammatory response and therefore seen as a favorable treatment
response [3,8].
Pseudoprogression is more common in patients with (+) methylated status. Methylation of O (6)-methylguanine-DNA
methyltransferase (MGMT) promoter leads to low MGMT expression
and show more sensitivity to TMZ. There is up to 91% probability
of pseudoprogression in patients with methylated MGMT and 59%
probability of early true tumor progression in patients without the
methylation status [3]. This increased sensitivity to TMZ in patients
with (+) methylated status is a good indicator of therapeutic response
and may indicate a better overall survival.
Therefore, it is important to recognize pseudoprogression as a
favorable treatment response. Patient can continue treatment when
pseudoprogression is recognized rather than stopping treatment
believing that the enhancement is due to early treatment failure.
Furthermore, if TMZ is discontinued erroneously, the new treatment
strategy may lead to decreased enhancement due to resolution of
pseudoprogression with false attribution to the efficacy of the new
treatment.
Biological imaging-related changes
Biological therapy can be used as treatments that exploit the
immune system to recognize and fight cancer cells. Glioblastoma
is associated with increased Vascular Endothelial Growth Factor
(VEGF) that results in highly angiogenic tumors with disorganized
vessels. The abnormal vessels have decreased vessel permeability with
localized hypoxia that induces a cycle of further increase in VEGF
[14]. By reducing angiogenesis, it is thought that perhaps the tumor would be limited by hypoxia and nutrient deprivation. However, increased hypoxia actually promotes angiogenesis, cancer cell
invasion, and possibly treatment resistance [14].
Bevacizumab (BEV) is approved for use in patients with
recurrent glioblastoma. BEV inhibits angiogenesis by acting as an
antibody that targets VEGF [4]. It is thought that in patients with
recurrent glioblastoma, BEV can “normalize” tumor vasculature
and blood-brain barrier and has measurable radiographic response.
Reduced contrast enhancement can be seen as early as day 1 after
start of therapy [14]. Fast reduction in tumor contrast enhancement
often occurs within days due to decreased vascular permeability and
improved edema [13]. On perfusion imaging, there is also decreased cerebral blood volume due to reduced vessel size [15]. Post-treatment decrease in contrast enhancement and edema may be seen in 25 to
60%of patients, but may not necessarily indicate true tumor reduction
[16]. This phenomenon of persistent viable tumor combined with
marked decreased in contrast enhancement on edema on MR after
starting BEV is termed pseudo response (Figure 7).
Additional explanations for pseudo response include viable hyper
cellular tumor, pseudo-infarct/infarct, atypical necrosis, metastasis,
gliomatosis phenotype, mix of tumor and treatment effect, and
resistance to therapy [14,17]. Eventually, glioblastoma treated with
angiogenics such as BEV will progress due to two theories of resistance.
Tumors may acquire the ability to evade angiogenic blockade or have a primary resistance to therapy [14]. Resistance to therapy can be due
to vessel co-option, mimicry, hypoxia-induced up regulation of other
angiogenic factor, among other mechanisms [14,17]. On the other
hand, studies show that BEV may not necessarily promote increased
remote risk of malignant glioma relapse [18].
Studies have found that BEV improves symptoms from mass
effect and quality of life, but does not improve the overall survival
rate [19]. Despite approval for recurrent glioblastoma, BEV may
not be beneficial in unselected populations. However, no validated
biomarkers exist for patient stratification at this time to help identify
the subset of patients most likely to benefit from BEV [14].
Alternative techniques such as diffusion-weighted imaging and
restriction-spectrum imaging have shown to be promising in defining
tumor response and non-enhancing tumor progression [20,21].
Contrast enhancement is a poor way to monitor tumor response
due to permeability of the blood-brain barrier. BEV is noted to
cause marked persistent areas of restricted diffusion in patients with
highly cellular tumors such as glioblastoma [22]. Therefore, restricted
diffusion can be used as a method to monitor treatment response after
radiation and chemotherapy [15]. This BEV-associated restricted
diffusion is thought to represent a form of radiation necrosis based
on pathology [15].
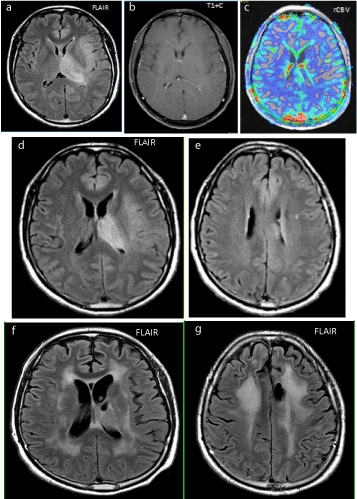
Figure 1
Figure 1
Figure 1a-c: At presentation, FLAIR hyperintense, but non-enhancing on post contrast T1W images and, non-hyperemic infiltrating glioma (no increase in rCVB).
Figure 1d-e: 2 years after whole brain radiation and boost to the left thalamus plus TMZ shows mild progression of perilesional and white matter FLAIR hyperintensity, also new mild generalized brain volume loss.
Figure 1f-g: 3 years after radiation with progression of atrophy and confluent FLAIR hyperintensity.
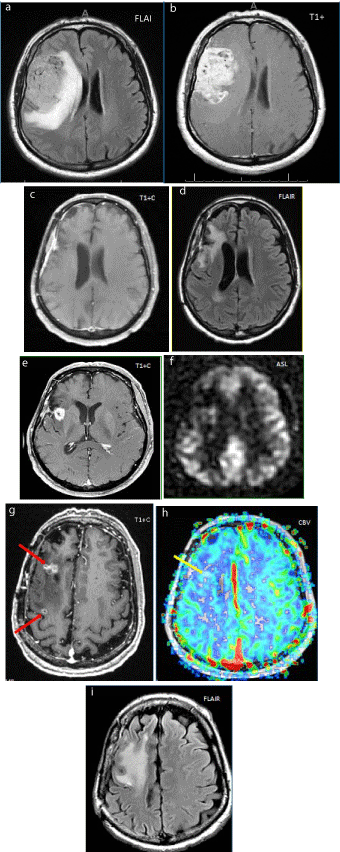
Figure 2
Figure 2
Figure 2a-b: Preoperative MRI shows FLAIR hyperintense and infiltrative GBM with prominent heterogeneous enhancement on post contrast T1Wimages.
Figure 2c-d: 2 years after surgery and completion XRT/TMZ. No significant FLAIR hyperintense or postcontrast T1W enhancement at surgical bed, which indicates absence of significant tumor residue.
Figure 2e-f: 3 years after surgery. New enhancing lesion on T1W postcontrast images, without increased perfusion on ASL;demonstrated to be radiation necrosis on pathology.
Figure 2g-i: 4.5 years after surgery. Newenhancing “soap bubble” distant foci (↑) on enhanced T1W images, not hyper-perfused on dynamic susceptibility contrast perfusion study (↑). Still on surveillance, but thought to be new distant foci of radiation necrosis.
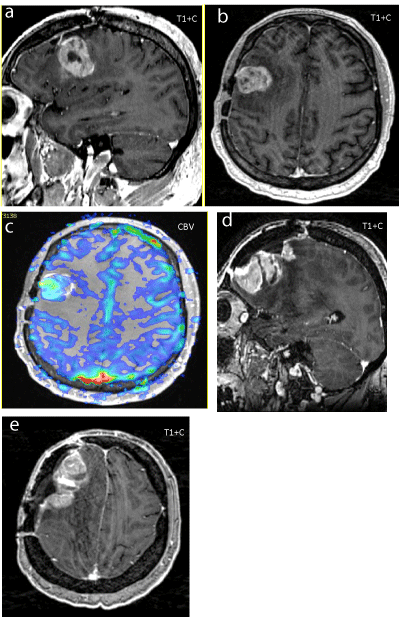
Figure 3
Figure 3
Figure 3a-c: Recurrent enhancing GBM (T1W) 18 months after total resection/XRT (60Gy)/TMZ, (no extra-axial lesions/clean surgical flap), underwent redo GBM resection.
Figure 3d-e: Follow up 11 months later, after GBM Resection x 2 + XRT boost (20 Gy) +TMZ, a collision extra-axial T1W enhancing meningioma in the resectionradiation site (at bone flap) is present.
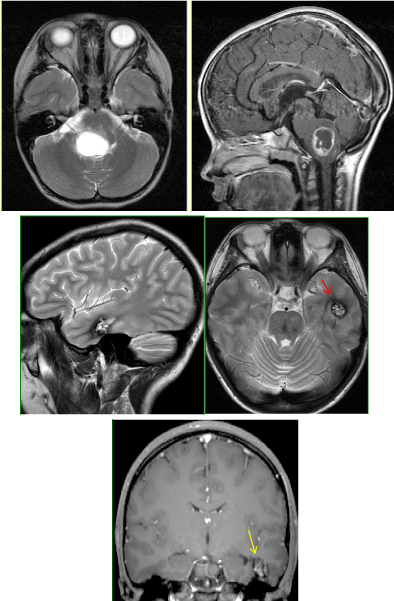
Figure 4
Figure 4
Figure 4a-b: Initial presentation of T2 hyperintense and peripherally enhancing T1W brainstem pilocytic astrocytoma. Received wide-field radiation.
Figure 4c-e: 10 years later: New lesion in the left temporal lobe consistent with cavernous malformation
(↑): Popcorn appearance with surrounding hemosiderin rim on T2
(↑): minimal/no enhancement on postcontrast T1W images.
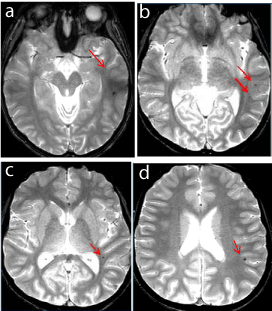
Figure 5
Figure 5a-d
15 years old status post infratentorial ependymoma resection and radiation 6 years ago. New tiny left temporo-parietal T2 hypointense foci in an otherwise unremarkable supratentorial brain. (↑)
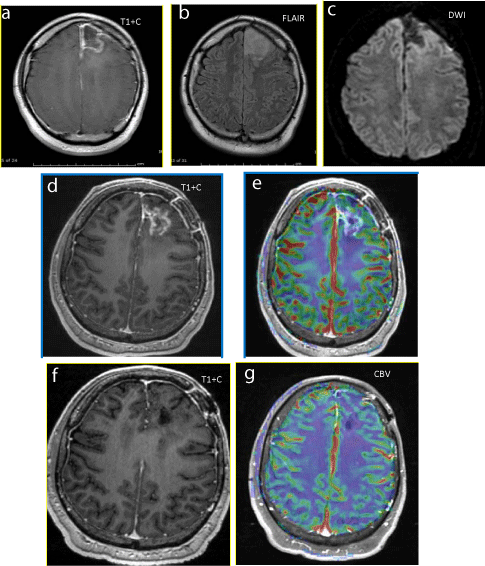
Figure 6
Figure 6
Figure 6a-c: Initial Postoperative images after GBM resection with no significant residue. No nodular enhancement on T1W. No surrounding FLAIR hyperintensity or restricted diffusion.
Figure 6d-e: 3 months after XRT-TMZ, new enhancing lesion on T1W images along the margins of the left frontal resection cavity without increased perfusion (no elevated rCBV).
Figure 6f-g: 9 months after XRT-TMZ, spontaneous resolution of enhancing, non-hyperperfused lesion.
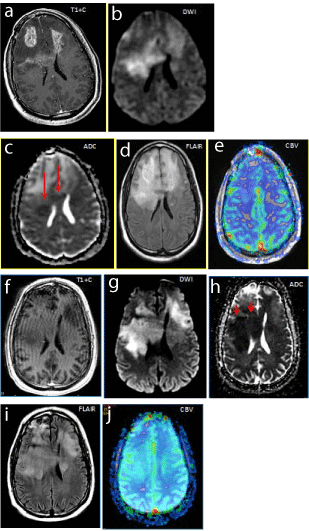
Figure 7
Figure 7
Figure 7a-e: Baseline recurrent bifrontal enhancing glioblastomapostcontrast T1 with, small focal areas of restricted diffusion (↑) on DWI/ADC ,extensive surrounding FLAIR hyperintensityand no significant increased perfusion (rCBV).
Figure 7f-j: 2 months after Bevacizumab, resolution of enhancement on postcontrast T1W, with worsening areas of restricted diffusion (↑) DWI/ADC. Worsening FLAIR hyperintensitywithout significant increase perfusion (rCBV).
Conclusion
In conclusion, radiation injuries to the central nervous are well documented and can occur within weeks in the form of vasogenic edema but often have additional delayed effects months to years later including leukoencephalopathy, cerebral atrophy, necrosis, induction of neoplasm and vasculopathy. Chemotherapy and biological therapy has been found to complicate the post treatment picture by pseudoprogression and pseudo response. By understanding the typical post-treatment responses seen in MR imaging, better clinical decisions can be made.
References
- Walker AJ, Ruzevick J, Malayeri AA, Rigamonti D, Lim M, Redmond KJ, et al. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol. 2014; 10: 1277-1297.
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J ClinOncol. 2010; 28: 1963-1972.
- Hyginodacruz LC, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of post treatment glioma. AJNR Am J Neuroradiol. 2011; 32: 1978-1985.
- Stone JB, Deangelis LM. Cancer-treatment-induced neurotoxicity-focus on newer treatments. Nat Rev ClinOncol. 2016; 13: 92-105.
- Welzel T, Niethammer A, Mende U, Heiland S, Wenz F, Debus J, et al. Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am J Neuroradiol. 2008; 29: 379-383.
- Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000; 217: 377-384.
- Ebi J, Sato H, Nakajima M, Shishido F. Incidence of leukoencephalopathy after whole-brain radiation therapy for brain metastases. Int J RadiatOncolBiol Phys. 2013; 85: 1212-1217.
- Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E. Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum--use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol. 2012; 198: 19-26.
- Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005; 26: 1967-1972.
- Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S. Radiationinduced meningioma. Neurosurg Focus. 2008; 24: E7.
- Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol. 2005; 26: 1158-1162.
- Bian W, Hess CP, Chang SM, Nelson SJ, Lupo JM. Susceptibility-weighted MR imaging of radiation therapy-induced cerebral microbleeds in patients with glioma: a comparison between 3T and 7T. Neuroradiology. 2014; 56: 91-96.
- Pope WB, Young JR, Ellingson BM. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. CurrNeurolNeurosci Rep. 2011; 11: 336-344.
- Lu-Emerson C, Duda DG, Emblem KE, Taylor JW, Gerstner ER, Loeffler JS, et al. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J ClinOncol. 2015; 33: 1197-1213.
- Hesselink JR, Barkovich MJ, Seibert TM, Farid N, Muller KA, Murphy KT, et al. Bevacizumab: radiation combination produces restricted diffusion on brain MRI. CNS Oncol. 2014; 3: 329-335.
- Wick W, Wick A, Weiler M, Weller M. Patterns of progression in malignant glioma following anti-VEGF therapy: perceptions and evidence. Curr Neurol Neurosci Rep. 2011; 11: 305-312.
- Mountzios G, Pentheroudakis G, Carmeliet P. Bevacizumab and micrometastases: revisiting the preclinical and clinical rollercoaster. PharmacolTher. 2014; 141: 117-124.
- Wick A, Dörner N, Schäfer N, Hofer S, Heiland S, Schemmer D, et al. Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann Neurol. 2011; 69: 586-592.
- Wang Y, Xing D, Zhao M, Wang J, Yang Y. The Role of a Single Angiogenesis Inhibitor in the Treatment of Recurrent Glioblastoma Multiforme: A Meta-Analysis and Systematic Review. PLoS ONE. 2016; 11: e0152170.
- Mong S, Ellingson BM, Nghiemphu PL, Kim HJ, Mirsadraei L, Lai A, et al. Persistent diffusion-restricted lesions in bevacizumab-treated malignant gliomas are associated with improved survival compared with matched controls. AJNR Am J Neuroradiol. 2012; 33: 1763-1770.
- Kothari PD, White NS, Farid N, Chung R, Kuperman JM, Girard HM, et al. Longitudinal restriction spectrum imaging is resistant to pseudoresponse in patients with high-grade gliomas treated with bevacizumab. AJNR Am J Neuroradiol. 2013; 34: 1752-1757.
- Farid N, Almeida-Freitas DB, White NS, McDonald CR, Kuperman JM, Almutairi AA, et al. Combining diffusion and perfusion differentiates tumor from bevacizumab-related imaging abnormality (bria). J Neurooncol. 2014; 120: 539-546.