Research Article
Prognostic Significance of Inflammatory Cytokines in Obese Patients with Endometrial Cancer Undergoing Robotic Versus Laparotomy Procedures
Dimitrov T1*, Gorchev G1, Tomov S1, Sarfraz A2, Kameliya1, Tsvetanova1, Tantchev L1, Malkodanski I1 and Ivanova I1
*Corresponding author: Todor Dimitrov, Department of Gynecological Oncology, Medical University of Pleven, 8A Georgi Kochev Blvd, 5800 Pleven, Bulgaria
Published: 07 Sep, 2016
Cite this article as: Dimitrov T, Gorchev G, Tomov S,
Sarfraz A, Kameliya, Tsvetanova,
et al. Prognostic Significance of
Inflammatory Cytokines in Obese
Patients with Endometrial Cancer
Undergoing Robotic Versus Laparotomy
Procedures. Clin Oncol. 2016; 1: 1076.
Abstract
Several biological mechanisms mediate the association between obesity and endometrial neoplastic
risk. The increased body mass index (BMI) with >30 kg/m2 is also associated with the long-term
maintaining of a high level of inflammatory processes, increased pro-inflammatory cytokines,
and acute-phase inflammatory proteins. In the practice of gynecologic surgeons, there is growing
interest in monitoring a group of cytokines as independent prognostic factors (such as markers
of aggressiveness and opportunity for better therapeutic influence on the neoplastic process). We
report on the monitoring of cytokine profiles of interleukin (IL)-8, tumor necrosis factor (TNF)-α
and their association with C-reactive protein (CRP) in the serum of patients with stage I endometrial
cancer treated with robotic-assisted laparoscopic hysterectomy (R) and traditional laparotomy/open
(O) surgical procedures. The patients’ age ranged from 46-81 years. Compared to the pre-surgical
specimens, the levels of IL-8, TNF-α and CRP were substantially higher in post-surgical specimens,
particularly at 3rd hour. Regardless of the pre- or post-surgical time points, the levels of these
cytokines/mediators have been significantly higher in morbidly obese patients with BMI >40 kg/m2.
It is conclude that the inflammatory cytokines and acute phase proteins contribute to the prognostic
assessment in the neoplastic disease, particularly in morbidly obese endometrial cancer patients.
Immune-sparing role of robotic-assisted surgery as compared to open surgery seems to be a better
treatment option for overweight women with early-stage endometrial cancer.
Keywords: Endometrial cancer; Obesity; Inflammatory cytokines; Robotic surgery; Prognosis
Introduction
Endometrial cancer is the most common form of gynecological malignancies. The main characteristics of Type I endometrial cancer patients are: endometrioid histology, low-grade, ~85% distribution, estrogen-dependent, obese body habitus, peri-menopausal age, risk factors (diabetes, polycystic ovary syndrome, nulliparity, and late menopause), genetic mutations (PTEN activation, KRAS mutation, and microsatellite instability) with relatively better prognosis [1]. Malignant neoplasms of the uterine mainly affect the endometrium and their increased incidence is frequently associated with triggering the pathogenetic mechanisms such as endocrine disorders, hypokinesia (reduced locomotor activity, obesity, hormone therapy), etc [2]. Overweight women with body mass index (BMI) >30 kg/m2 face three-fold higher risk of developing endometrial cancer as compared to the normal weight women [3]. In the post-menopausal period, obesity leads to conversion of androgens, increase in serum bioavailability of estrogens that are not balanced by progesterone, which in turn, promotes the mitogenic activity of endometrial cells [4-6]. Obesity is also associated with maintaining a continuous high level of inflammatory processes, thereby increasing the levels of pro-inflammatory cytokines and acute-phase inflammatory proteins [7-9]. Surgery is a controlled injury, and in the practice of gynecologic surgeons, there is growing interest for potential role(s) of a group of cytokines that may serve as independent prognostic factors. These cytokines may potentially be identified as markers of the disease aggressiveness and provide opportunity for therapeutic influence on the neoplastic process because cytokines are immunological products, which are signs of biological balance and can potentially alter the programmable cell death [10,11]. Hence, comprehensive assessment at immunological level of the distortions in intracellular metabolism forms the basis for a differential approach to some of the predictions concerning the individual differences in neoplastic aggression, the influence of anesthesia and surgical intervention, post-operative recovery, and prognosis. This is even more relevant for overweight patients with endometrial cancer, given the fact that obesity is becoming a global medico-social problem. Therefore, in view of the above, we studied the prognostic significance of monitoring profiles of some of the key inflammatory cytokines [interleukin (IL)-8 and tumor necrosis factor (TNF)-α)] and its association with C-reactive protein (CRP) in overweight patients with stage I endometrial cancer treatment by robotic-assisted laparoscopic hysterectomy (R) and traditional laparotomy/open (O) surgical procedures.
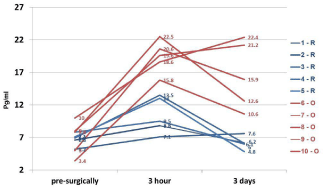
Figure 1
Figure 1
Measurement of IL-8 level in the serum of women with stage I
endometrial cancer treated with robotic-assisted laparoscopic (R) and
conventional laparotomy/open (O) surgical techniques.
Material and Methods
At our primary single institution, 97 women diagnosed with stage I endometrial cancer for this study. The patients were divided into two groups based on the individual diagnostic characteristics and their BMI, and surgical approach/procedures performed (i.e., O vs. R). During the course of the pre-operative clinical diagnosis, 68 patients were identified with an extremely high BMI (>40 kg/m2). The surgical management of early-stage endometrial cancer patients were accomplished through our standard operative procedures optimized for the traditional laparotomy/open (O) and the newer state-of-the-art minimally invasive (MIS) approach, i.e., roboticassisted laparoscopic surgery (R). All surgeons who participated in the study were competent in both surgical procedures. Patients’ demographics, clinico-pathological, and peri-operatives data were contemporaneously abstracted from the medical records as an ongoing quality initiative. This use of clinical specimen and corresponding retrospective data used in this study was approved by the primary institutional review board. For dynamic monitoring, patients blood samples were collected pre-operatively, and at the 3rd hour and 3 rd day, post-operatively. Serum specimens thus obtained were stored at -20oC before use. The levels of IL-8, TNF-α, and CRP were measured utilizing the respective human ELISA assay kits (Diaclone SAS, Besancone Cedex, France) accordingly to the manufacturer’s protocols. The samples were read at 450 nm optical density on an ELISA Microplate Reader (Model 2100 Stat Fax, Awareness Technology, Inc, Ramsey, MN, USA).
Results
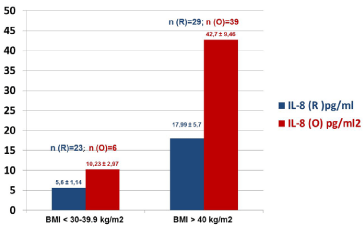
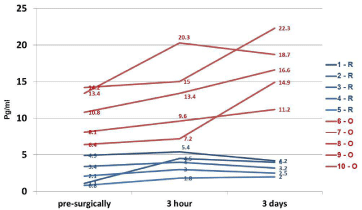
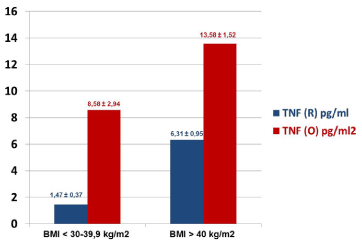
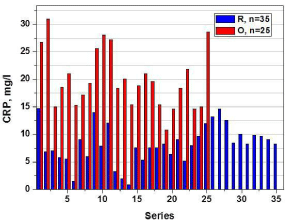
The patients’ age ranged from 46-81 years (n=97). The comparative characteristics of the dynamic monitoring of serum levels of IL-8 in the two surgical group of the patients are presented in (Figure 1). It is evident that compared to the pre-surgical, the levels of IL-8 in the specimens collected at the 3rd hour post-operatively are much higher in O group (in red) and remained higher even after day-3, post-operatively. In contrast, the R group (in blue) of patients was found to have relatively lower levels of IL-8 in serum specimens collected at all time-points (Figure 1). (Figure 2) summarizes the data on IL-8 levels (at 3rd hour, post-operatively) in accordance with BMI of the study patients and the type of surgical procedures performed. In the lower BMI group of patients (left panel), the IL-8 levels were found to be nearly 2-fold higher in O group specimens as compared to the R group. Interestingly, this phenomenon was even more obvious in the patients with BMI >40 kg/m2 where IL-8 levels were higher in the O groups of samples as compared to the R group (Figure 2). Furthermore, morbidly higher BMI of patients was clearly associated with elevated levels of IL-8 (about 3 to 4-fold) regardless of the surgical approach/interventions (Figure 2). Next, in view of the multifactorial significance of TNF-α, the dynamic serum level of TNF-α was measured in these specimens. As shown in Figure 3, TNF-α level was relatively higher in the open surgery group of patients at all the time-points (in red) as compared to the robotic group of patients (in blue). Notably, the R group of specimens at all time-points exhibited minimum deviations as compared to the O group. Also, a slight reduction is observed in the levels of TNF-α (close to baseline values) after the 3rd day following the R surgery, which lacked in the O group, (Figure 3). In fact, three of the women operated with O technique, TNF-α continued to rise on the 3rd day after the intervention. These patients also have had clinically proven post-operative complications (infection, dehiscence, and intestinal obstruction). The results on the measurement of TNF-α levels (at 3rd hour, post-operatively) in accordance with BMI of the study patients and the type of surgical procedures performed are shown in (Figure 4). In the lower BMI group of patients (left panel), the TNF-α levels were higher (more than 5-fold) in O group specimens as compared to the R group. Likewise, the TNF-α levels were consistently higher in both surgical groups in patients with BMI >40 kg/m2. The level TNF-α was more than double in the open group as compared to the robotics group of morbidly obese patients (Figure 4). We then measured the CRP level in the patients’ specimens collected at the 3rd hour postoperatively in both surgical groups. Individual patients’ CRP levels are shown in Figure 5. Based on the omparative analyses, the average values in robotic technique was CRP = 7.906 mg/L) (which turns out to be within the normal range of <10 mg/L). However, a significant increase in the average CRP level was observed in the open surgery group (Δ CRP = 19.886 mg/L), suggesting the persistence of an inflammatory response, (Figure 5).
Figure 2
Figure 2
Cumulative levels of IL-8 in the BMI stratified specimens collected
at 3rd hour post-operatively from women with stage I endometrial carcinoma,
operated with robotic-assisted laparoscopic (R) and conventional laparotomy/
open (O) surgical approaches.
Figure 3
Figure 3
Measurement of TNF-α level in the serum of women with stage
I endometrial cancer treated with robotic-assisted laparoscopic (R) and
conventional laparotomy/open (O) surgical techniques.
Figure 4
Figure 4
Cumulative levels of TNF-α in the BMI stratified specimens
collected at 3rd hour post-operatively from women with stage I endometrial
carcinoma, operated with robotic-assisted laparoscopic (R) and conventional
laparotomy/open (O) surgical approaches.
Discussion
This study demonstrates that profiling of immunological
cytokines (such as IL-8, TNF-α, and CRP) contributes to the
prognostic assessment in neoplastic endometrial cancer. Our data
also demonstrates the immune-sparing role of the MIS (roboticassisted
laparoscopic hysterectomy) as a better treatment option for
overweight women with stage I endometrial cancer as compared to the
traditional laparotomy/pen surgical approach. Endometrial cancer is
the frequent gynecologic malignancies, and most patients present
with early-stage disease. The common symptoms of this disease are:
vaginal bleeding after menopause, bleeding between periods, an
abnormal, watery or blood-tinged discharge from the vagina, and
pelvic pain, etc. Hence, the disease prognosis and timely detection
is critical in the successful management of such patients. Current
standard treatment of patients with endometrial cancer is surgery (i.e.,
hysterectomy, salpingo-oophorectomy, and/or lymphadenectomy,
preferably MIS), followed by chemotherapy, radiation and/or
hormone therapy (depending on the various clinico-pathological and
peri-operative factors). In addition to the above noted facts, tumor
markers are being increasingly recognized as important tools for early
diagnosis, prognosis, therapy response and monitoring for patients
with endometrial cancer. It has been widely recognized that IL-8
affects the movement of leukocytes and macrophages (chemotaxis,
migration, respiratory burst) by promoting antimicrobial activity.
The extent of the inflammatory state of the body is also caused
by the level of proinflammatory cytokine such as IL-8, which is
associated with the angiogenic effect on thymic endothelial cells that
is responsible for cell survival rate [12-14]. Our data clearly suggest
that post-operatively IL-8 levels are up-regulated in patients with
early-state endometrial cancer, particularly in much higher levels in
the morbidly obese cases.
Another role in the progress of inflammatory immune processes,
cellular apoptosis, and malignancies is attributable to TNF-α. Along
with other key interleukins (such as IL-6, IL-8, and IL-10, etc.), this
cytokine is also involved in both the acute and chronic inflammatory
processes. Various immune epithelial and tumor cells take part in the
production of TNF-α, which is accumulated intracellularly, forming a
non-covalent trimeric bond, as it expresses on the cell surface. When
bound on the cell surface, TNF-α may form lysis with the tumor or
virally-infected cell. The separation of the membrane-bound TNF-α
leads to feedback about the activation or delay in the production
of TNF-α itself and of other biologically active cytokines [15,16].
Our observations on the measurement of TNF-α support the fact
that post-operatively this cytokine is also up-regulated endometrial
cancer, particularly in much higher levels in the morbidly obese and
those treated with open surgical procedure. The activation of TNF-α
triggers the activation or suppression of a number of inflammatory
processes, vascular permeability, hemorrhagic and necrotic
deviations, tumor-associated vascularisation, anti-tumor treatment
and further spread of the neoplastic process.
The increase in IL-8 and TNF-α entails an increase in CRP
levels, which is synthesized by the liver in response to inflammatory,
infectious, neoplastic, traumatic or autoimmune processes. CRP is a
pentameric glycoprotein, commonly known as acute-phase protein.
This protein is often used as apoptosis and necrosis marker (with
normal value in blood plasma of <10 mg/L). It binds with lipophosphatidylcholine
of the cell membranes, with further activation
of the complement cascade, phagocytosis, fibrinogen synthesis and
closing of the pathogenetic circle while maintaining the inflammatory
process [13,16,17]. As noted above, the CRP levels were significantly
upregulated across the board in endometrial cancer patients treated
with open laparotomy surgical group (post-operatively). This CRP
increase was associated with the increase in IL-8 and TNF-α levels,
particularly at 3-hours, post-operatively. The MIS is a method to reduce
the morbidity of surgery and has been shown in general to reduce
blood loss, complications, post-operative pain and hospital length-ofstay
compared with traditional laparotomy approach [18]. In order
to treat the disease, surgeons balance complications and invasiveness
with clinical outcomes in order to determine which techniques are
best. The widespread adoption of robotic surgery (most updated
form of MIS) for gynecologic indications during the past decade
have been encouraging Its development and utilization addresses
many of the limitations of traditional laparoscopy instruments by
restoring dexterity and intuitive instrument movement, 3-D vision,
ergonomics, and autonomy.
Figure 5
Figure 5
Measurement of CRP level in the serum of women with stage I
endometrial carcinoma treated with robotic-assisted laparoscopic (R; n=35)
and conventional laparotomy/open (O; n=25) surgical techniques at 3rd hour,
post-operatively.
Conclusion
We thus conclude that the inflammatory cytokines and acute phase proteins contribute to the prognostic assessment in the case of neoplastic diseases, particularly in morbidly obese endometrial cancer. Furthermore, the immune-sparing role of robotic-assisted surgery as compared to open surgery is demonstrated as a better treatment option for overweight women with early-stage endometrial cancer. Our data re-confirms that MIS (robotic surgery) reduces the so called “cytotoxic burst”, which presupposes the decrease of portoperative risk of hospitalization, complications, and potentially intensive care encounters.
References
- Bregar A, Robison K, Dizon DS. Update on the chemotherapeutic management of endometrial cancer. Clin Adv Hematol Oncol. 2014; 12: 659-665.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms. 2016; 13.
- Dossus L, Lukanova A, Rinaldi S, Allen N, Cust AE, Becker S, et al. Hormonal, metabolic and inflammatory profiles and endometrial cancer risk within the EPIC cohort-a factor analysis. Am J Epidemiol. 2013; 177: 787-799.
- Pavelka JC, Ben-Shachar B, Fowler JM. Morbid obesity and endometrial cancer: Surgical, clinical and pathologic outcomes in surgically managed patients. Gynecol Oncol. 2004; 95: 588-592.
- Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: Have they different risk factors? J Clin Oncol. 2013; 31: 2607-2618.
- Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity and endometrial cancer risk: results from the Netherlands cohort study. Int J Gynecol Cancer. 2006; 2: 492.
- Grosen EA, Yamamoto RS, Ioli G, Ininns WK, Gatanaga T, DiSaia PJ, et al. Blocking factors (soluble membrane receptors) for tumor necrosis factor and lymphotoxin detected in ascites and released in short-term cultures obtained from ascites and solid tumors in women with gynecologic malignancy. Lymphokine Cytokine Res. 1992; 11: 347-353.
- Guo Y, Nemeth J, O’Brien C, Susa M, Liu X, Zhang Z, et al. Effects of siltuximab on the IL-6 induced signaling pathway in ovarian cancer. Clin Cancer Res. 2010; 16: 5759-5769.
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl Acad Sci. USA 1994; 91: 4854-4858.
- Smith HO, Stephens ND, Qualls CR, Fligelman T, Wnag T, Lin CY, et al. The clinical significance of inflammatory cytokines in primary cell culture in endometrial carcinoma. Mol Oncol. 2013; 7: 41-54.
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008; 8: 887-899.
- Mantovani G, Maccio A, Mura L, Massa E, Mudu MC, Mulas C, et al. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med. (Berl) 2000; 78: 554-561.
- Michaud A, Drolet R, Noel S, Paris G, Tchernof A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism. 2012; 61: 689-698.
- Nash M, Ferrandina G, Gordinier M, Loercher A, Freedman R. The role of cytokines in both the normal and malignant ovary. Endocr Relat Cancer. 1999; 6: 93-107.
- Mathew SJ, Haubert D, Krӧnke M, Leptin M. Looking beyond death: A morphogenetic role for the TNF signaling pathway. J Cell Sci. 2009; 122: 1939-1946.
- Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+regulatory T cells in gastric cancer. Int J Cancer. 2008; 122: 2286-2293.
- Modugne F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: A hypothesis. Cancer Epidemiol Biomarkers Prev. 2005; 14: 2840-2847.
- Holloway RW, Patel SD, Ahmad S. Robotic surgery in gynecology. Scand J Surg. 2009; 98: 96-109.