Research Article
The Effect of Myometrial Invasion Pattern to Stage in Endometrial Cancer
Uslu E1, Yalcin OT1, Kabukcuoglu S2*, Ozalp SS1 and Oge T1
1Department of Obstetrics and Gynecology, Eskisehir Osmangazi University School of Medicine, Turkey
2Department of Pathology, Eskisehir Osmangazi University School of Medicine, Turkey
*Corresponding author: Kabukcuoglu S, Department of Pathology, Eskisehir Osmangazi University School of Medicine, Eskisehir, Turkey
Published: 29 Jul, 2016
Cite this article as: Uslu E, Yalcin OT, Kabukcuoglu
S, Ozalp SS, Oge T. The Effect of
Myometrial Invasion Pattern to Stage in
Endometrial Cancer. Clin Oncol. 2016;
1: 1048.
Abstract
Aim: Myometrial invasion (MI) is one of the prognostic factors in endometrial cancer (EC). It has
been argued that myometrial invasion pattern (MIP) is associated with prognosis. Our aim is to
evaluate MIP in patients with EC.
Materials and Methods: The patient who had total abdominal hysterectomy, bilateral salpingooophorectomy,
in fracolicomentectomy, pelvic-paraaortic lymphadenectomy, cytological sampling
and also has a result of EC in postoperative pathology between May 2014- October 2015 in our
clinic were included. Specimens were hystopathological classified as properly limited (pushing, PL),
diffusely infiltrative (DI), adenomyozisinvolment by EC (A), microcystic, elongated and fragmented
(MELF) groups by the same pathologist. The relationship between the MIP and stage and grade of
endometrial cancer were evaluated.
Results: One hundred patients was operated and mean age was 57.4 ± 9.32 years. PL, DI, A, MELF
patterns were observed in 41,14,17,28 patients respectively. Distribution of myometrial invasion
patterns to stages, MELF pattern was observed mostly in Stage 3(n=12, 42.9%). Properly limited
(n=30, 73.2%,), DI(n=5, 35.7%) and AL(n=9 , 52.9%) patterns are observed mostly in Stage
1A(P=0,001). Moreover, grade 1 was observed in most of the PL (n=6, 75%), andgrade 3 was
observed in most MELF pattern in endometrial cancer (n=10, 52.6%)(P=<0,001).
Conclusions: MELF pattern was mostly observed in patients with high grades and stages of
endometrial cancer. Properly limited pattern was mainly observed in low grade and stage of
endometrial cancer. Further studies are needed to evaluate the clinical significance of this
observation.
Keywords: Endometrial cancer; Myometrial invasion pattern; Stage; Immunohistochemistry
Introduction
EC, with a lifetime risk of 2.5%, is the most common gynecologic cancer [1]. Despite good clinical outcome, a small but significant number of patients may experience recurrence, and it has not been possible to predict which patients are at increased risk. Prognostic factors for EC are patient age, tumor grade, histological subtype, depth (MI), extra uterine extension (EUE) and lymph node (LN) metastasis [2]. MI is one of the most important prognostic factor [3-5]. In recent years, the MI pattern has been proposed as a potential prognosis predictor. EC shows different MI patterns, PL, DI, A, MELF and adenoma malignum (AM) [6-8]. Murray et al. [7] first described MELF invasion pattern in 2003. Clinical significance of MELF pattern of invasion remains unclear, although it is reported to be associated with distinct changes in the invasive glandular tissues and a high frequency of lymphovascular space invasion (LVSI) [3,7,9,10]. In this study, we aimed to evaluate the relationship between the MIP and stage and grade of EC.
Materials and Methods
The patient who had total abdominal hysterectomy, bilateral salpingo-oophorectomy, in
fracolicomentectomy, pelvic-paraaortic lymphadenectomy, cytological sampling and also has a
result of EC in postoperative pathology between May 2014- October 2015 in Eskişehir Osmangazi
University Hospital were included. All cases were classified according to International Federation
of Gynecology and Obstetrics (FIGO) staging system [11]. Fallopian tube sampling was made
according to SEE-FIM protocol. Tissue specimens were formalin fixed, paraffin embedded and
subsequently sectioned at 4μm thickness. The immunohistochemical studies were performed using
a standart procedure on an automated immunostainer (Ventana ES,
Ventana Medical Systems, Inc, AZ, U.S.A). Liquid rabbit monoclonal
antigens were used as primary antigen. Immunohistochemical
staining for estrogen receptor (ER), progesterone receptor (PR),
Paired box 8 (PAX8), caudal type homeobox 2 (CDX2), CK19,
E-cadherin, beta catenin (β-cat), TP53 was performed on selected
sections. Appropriate positive control procedures were performed
with satisfactory staining in the whole study. DAB chromogen was
used. The sections counter stained with Mayer’s hematoxyl in and
the sections were dehydrated, cleared, mounted. Those specimens
with histopathologic examination grade, myometrial invasion of
myometrial invasion pattern (Pl, DI, A, MELF, AM), the presence of
vascular invasion and extra uterin extension (EUE) were determined
by the same pathologist.
Statistical analyses
All statistical analyses were performed using SPSS (Statistical
Package of Social Services, Chicago, IL, USA) for Windows version
21. Data were analysed according to Pearson Chi – square, Shapiro
Wilk normality test, ANOVA and Tamhane test. Probability values
less than 0.05% were considered statistically significant.
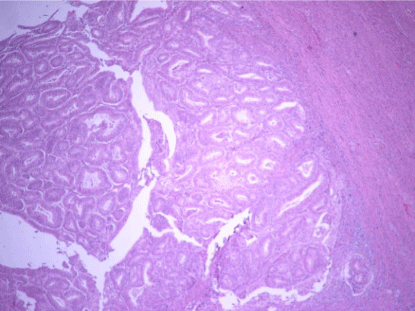
Figure 1
Figure 1
Low grade EC, PL pattern (H-E x200). Cases that seen PL,
neoplastic glands were found to create pushing infiltration on myometrium.
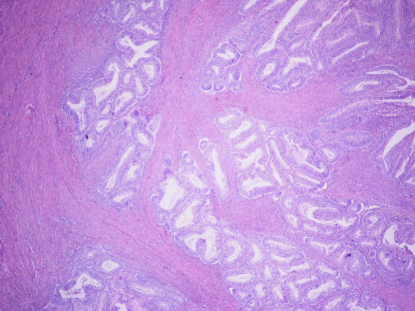
Figure 2
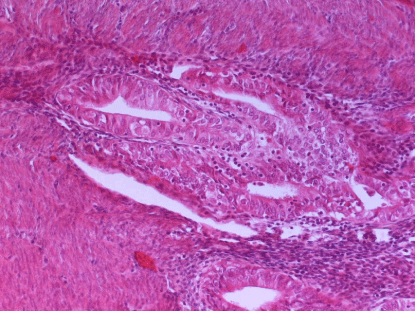
Figure 3
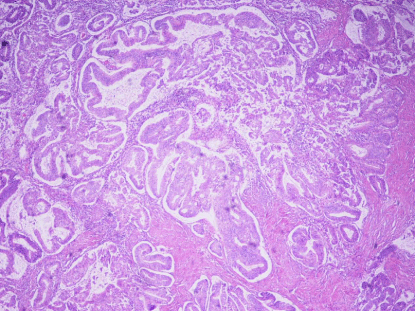
Figure 4
Figure 5
Results
In total, 102 consecutive cases of uterine ECwere identified. One
hundred and two patients were operated and mean age was 57.4 ±
9.32 years. Stage 1A, 1B, 2, 3A, 3B, 3C were observed in 48 (47%),
29 (28%), 7 (7%), 11 (11%), 1(1%), 6(6%) patients respectively. As
mentioned stage distribution of patients was statistically significant,
though may be to stage 3A, 3B and 3C are taken together as stage
3(18 patients, 17%). Grade 1, 2, 3 were observed in 8 (8%), 75 (73%),
19 (%18) respectively. Properly limited, DI, A, MELF patterns
were observed in 41 (40%), 14 (14%), 17 (17%), 28 (27%) patients
respectively. Two patients’ MIP are unidentified. Several cases
displayed more than one pattern of invasion, and the predominant
type of invasion was accepted in each case. Diagnostic criteria for
the various patterns of myoinvasion were as follows: PL(Pushing):
Infiltration that was marked by a large swath of neoplastic glands that
appear to push into the underlying myometrium (Figure 1).
DI: Single to small groups (3 or less) of glands with irregular gland
contours, with or without a desmoplastic stromal response (Figure 2).
A: Adenomyosisinvolment by Endometroid cancer (Figure 3).
MELF
Microcystic, elongated, and/or slit-like glands, with clusters
or individual tumor cells, which often appeared squamoid or
eosinophilic. Frequently, there was an accompanying loose myxoid,
mixed inflammatory reaction (Figure 4).
AIM
Myometrial involvement of cervical minimal deviation
adenocarcinoma (Figure 5). Stage and myometrial invasion patterns
were compared and MELF pattern is mostly seen in stage 3, other
patterns are mostly seen in stage 1A. This comparison found to be
statistically significant (P=0,0003) (Table 1). A comparison of grade
and myometrial invasion patterns is shown in (Table 2). Properly
limited pattern is mostly seen in grade 1, MELF pattern is mostly seen
in grade 3 (P=0,0034). There were 24 patients with focal squamous
differentiation, 6 patients with focal mucinous differentiation, 1
patient with neuroendocrine differentiation and 71 patients had no
diferentation.MIP and differentiations were compared and not found
to be statistically significant (P=0,307). A comparison of MIP and
LVSI is shown in (Table 3). Lymphovascular invasion was observed
in all MELF pattern patients and most of them made lymphovascular
invasion creating a group of cells. Properly limited, DI and A patterns
made lymphovascular invasion without creating a group of cells
(P=0,000).There was no significant difference in terms of the mean
age and MIP (ANOVA, P=0,366). The mean age and stage were
compared and found statistically significant (P=0,004). Stage 1A of
average age 55, Stage 1B of average age 61. Stage and grade compared
and found statistically significant (P=0,014) (Table 4). All of the Grade
1 was found in stage 1A. Grade 2 was observed most common in all
stages. Immunohistochemical staining for ER, PR, PAX8, CDX2,
CK19, E-cadherin, β-catenin, TP53 was performed. Tissue samples
divided into the groups according to percentage of stained cells.
Staining pattern of ER
Ninty seven samples stained by ER. Nuclear staining was observed
in 3 samples at 1-10%, 13,4% 13 of samples at 11-50%, 81% 79 of
samples at 51-100%. Two samples were negative.
Staining pattern of PR
Ninty seven samples stained by PR. Nuclear staining was observed
in 5 samples at 1-10%, 22,6% 22 of samples at 11-50%, 67% 65 of
samples at 51-100%. Five samples were negative.
Staining pattern of PAX8
Of the 87 samples were stained by PAX8. Three samples were
no staining. Three samples showed focal minimal nuclear staining,
33,7% 30 samples showed focal moderate staining, 51,7% 46 samples
showed diffuse heterogen staining, 8,9% 8 samples showed diffuse
nuclear staining.
Staining pattern of CDX2
CDX2 showed focal staining in morular metaplasia and squamous
differentiation areas and some cylindrical cells. CDX2 was positive in
ER and PR negative areas. Of the 93 samples were stained by CDX2.
Fourty two percent 39 of samples showed focal nuclear staining, 1
sample showed diffuse staining. Fifty seven percent 53 of samples
were negative.
Staining pattern of CK19
Of the 93 samples were stained by CK19. Two samples showed at
1-20%, 40% 37 samples showed at 21-50%, 29% 27 samples showed at
51-80% and 28% 26 samples showed at 81-100% staining. One sample
was negative.
Staining pattern of E-cadherin
Most of the cells are surrounded by E-cadherin staining. Of the
93 samples were stained by E-cadherin. Twenty eight percent 26
of samples showed heterogeneous membranous staining, 70% 66
samples diffuse membranous staining. One sample was negative.
Staining pattern of β-cathenin
β-cathenin showed membranous and cytoplasmic staining. In
squamous differentiation areas, nuclear staining was also observed.
Of the 47 samples were stained by β-cathenin. Membranous staining
observed in 85% 40 samples. Membranous, cytoplasmic and nuclear
staining in 6 samples were observed. One sample was negative.
Staining pattern of TP53
Of the 96 samples were stained by TP53. Fourty five percent 43
of samples showed at 1-10%, 11% 11 samples showed at 11-50%, 8%
8 samples showed at 51-100% staining. Thirty five percent of samples
were negative.
There were no statistical significant differences among
immunohistochemical staining patterns and MIP (Pearson χ2-test,
P>0, 05).There was statistical significant difference between TP53
staining rate and stage. When staining percent more compared,
negative staining was observed mostly stage 1A, 1-10% staing mostly
observed in stage 1B. There were no statistical significant differences
among immunohistochemical staining patterns of ER, PR, PAX8,
CDX’, CK19, E-cadherin,β-catheninand stage (Pearson χ2-test,
P>0,05).
Table 1
Table 2
Table 3
Table 4
Discussion
Endometroid cancer has good clinical outcome, on the other hand
some cases have recurrence or methastasis. Prognostic factors for EC
are patient age, tumor grade, histological subtype, depth of MI, EUE
and LN metastasis [2]. In a study in which 513 patients participated
Nofech-Moses et al. [12], showed that lymphovascular invasion an
important predictor parameter for distant recurrence in early-stage
EC. Guntupalli et al. [13] made a study with 628 patients who had
systematic lymphadenectomy, 196 of patients had lymphovascular
invasion and 66 of them had nodal metastases which was found
statistically significant. Grading the LVSI is indicated in the study of
Hachisug et al. [14] 303 patients were examined, grading of LVSI was
found to be an important histologic prognostic variable. The severe
degree of LVSI also was found to be a good indicator of lymph node
metastasis. In our study 75% of cases had a LVSI and %30 of them
made a cell groups (P=0,000). Properly limited group at least LVSI.
There are limited studies about MIP. Murray et al. [7] followed
115 EC cases with MI and investigated the MELF pattern exists or
not. In cases of lymphovascular invasion, commonly MELF pattern
associated with, including fibromyxoid reaction, death and recurrence
was more followed. Stewart et al. [10] examined 133 cases of EC and
in 27 of them were MELF positive. MELF-positive patients often focal
mucinous differentiation and lymphovascular space invasion was
observed. Pavlakiset al. [9] searched 351 patients. Lymph node-positive
patients, positive for MELF 53.84% was observed. MELF negative
of positive lymph nodes were seen in 6,97% and it was statistically
significant. Quick et al. [8] examined 324 patients, 98 of them had MI.
MIP were separated as infiltration of glands, MELF, impulsive and
AM. MI that tumors with infiltration of the glands were high-grade,
lymphovascular invasion and recurrence were found statistically
more frequent. In 2013 they made a compilation, it was important
to measure the depth of MI and MIP. They also suggested that there
may be a prognostic factor in certain patterns. MELF pattern was seen
in lymphovascular invasion were reported more frequently. It also
supports the study of Hertel et al. [15] 80 well differentiated of EC
with lymph node metastasis were investigated. Tumors with MELF
pattern had more lymph node metastasis, which was statistically
significant. Ismiil et al. [16] found that when the carcinoma invades
to adenomyosis, it increases the MI depth. In addition, although
rare, there are 2 studies about endometrial intraepithelial serous
carcinoma develop from adenomyosis [17,18]. In Orejuell et al.
[19] investigated of ER, PR, COX-2 immunohistochemical staining
patterns of normal endometrium, endometrial hyperplasia and EC.
COX-2 expression was more observed in hyperplasia and cancer but
statistically significance not found. Staining insamples of EC and there
was no significant difference in terms of grade and stage. Intensity
of ER and PR staining was normal and hyperplastic endometrium
much more than EC. Stewart et al. [20] showed in 21 patients with EC
patients. Conventional pattern tumors showed intensemembranous
staining by E-cadherin, hormone receptor activity and vimentin
positivity. MELF type of MI characterized by strong CK7 expression,
usually negative for hormone receptors and decreased expression
of E-cadherin. Stewart et al. [21], observed basal and apical CK19
staining in proliferative endometrium; strong in the conventional
type of large tumors cloth and around there is weak staining. In the
study, also the strong staining pattern was observed in MELF. CK19
is useful determining of myometrial and lymphovascular invasion.
CK19 is a selective estrogen receptor modulator and in our study,
there was no correlation between the stage with CK19 staining. An
actin-binding protein fascin, increases migratory capacity both in
normal and neoplastic cells. Kabukcuoglu et al. [22] investigated
the fascin staining patterns in28 proliferative and hyperplastic
endometrium and 43 cases of EC. The study supported the dynamic
role of actin bundling protein fascin in generating and maintaining
endometrial neoplasms. It also showed that in the development of
neoplasia, stromal fascin expression decreases but epithelial fascin
expression up-regulates. Gun et al. [23] supported also this data.
Stewart et al. [24], examined 28 EC cases by fascin and CK-7 staining.
Focal fascin reactivity in classic glandular component, was observed.
MELF pattern showed strong fascin immunoreactivity Observation
of increased fascin expression in MELF pattern, is suggestive of active
tumor invasion.
E-cadherin expression examined in 21 normal, 17 hyperplastic
endometrium and 104 EC, methylation of E-cadherin promoter
genes has been observed to be associated with the formation of
tumor invasive capacity [25]. In our study there was no significant
relationship between E-cadherin with MIP and stage. Yemelyanov
et al. [26], showed PAX-8 expression in EC, endometrial hyperplasia
and normal endometrium. PAX-8 expression has been observed also
in adenocarcinoma. Faucegli et al. [27], 228 patients were compared
according to the PAX-8 in high-grade, lymphovascular space invasion
positive and type 2 tumors were showed significantly more intense
PAX8 staining. Their 5-year disease-free survival rate was significantly
decreased. In our study, we also observed down regulation and/or up
regulation of PAX8 expression in EC patients. In our opinion intensity
of PAX8 expression may be related differentiation and anaplasia.
Dobrzyck et al. [28], 98 patients with EC have investigated the
relationship between p53 and bcl-2 expression of immunochemical
stage and survival.BCL-2 expression was observed more frequently
in the early stages. In this study, expression of TP53 staining percent
when compared with the stage, negative staining frequentlyassociated
with stage 1A and 1-10% staining is observed frequently in stage 1B.
Conclusion
As a result, there are some findings about MIP can affect the stage in EC. These findings should be investigated by examining the larger patient groups about the effect with pattern alone or multifunctional.
References
- Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA: A cancer journal for clinicians. 1996; 46: 5-27.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. 1983; 15: 10-17.
- Euscher E, Fox P, Bassett R, Al-Ghawi H, Ali-Fehmi R, Barbuto D, et al. The pattern of myometrial invasion as a predictor of lymph node metastasis or extrauterine disease in low-grade endometrial carcinoma. A J surgi pathol. 2013; 37: 1728-1736.
- Goff BA, Rice LW. Assessment of depth of myometrial invasion in endometrial adenocarcinoma. Gynecol Oncol. 1990; 38: 46-48.
- Lee KB, Ki KD, Lee JM, Lee JK, Kim JW, Cho CH, et al. The risk of lymph node metastasis based on myometrial invasion and tumor grade in endometrioid uterine cancers: a multicenter, retrospective Korean study. Ann Surg Oncol. 2009; 16: 2882-2887.
- Longacre TA, Hendrickson MR. Diffusely infiltrative endometrial adenocarcinoma: an adenoma malignum pattern of myoinvasion. Am J Surg Pathol. 1999; 23: 69-78.
- Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. International journal of gynecological pathology: Int J Gynecol Pathol. 2003; 22: 324-333.
- Quick CM, May T, Horowitz NS, Nucci MR. Low-grade. low-stage endometrioid endometrial adenocarcinoma: a clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. International journal of gynecological pathology : Int J Gynecol Pathol. 2012; 31: 337-343.
- Pavlakis K, Messini I, Vrekoussis T, Panoskaltsis T, Chrysanthakis D, Yiannou P, et al. MELF invasion in endometrial cancer as a risk factor for lymph node metastasis. Histopathology. 2011; 58: 966-973.
- Stewart CJ, Brennan BA, Leung YC, Little L. MELF pattern invasion in endometrial carcinoma: association with low grade, myoinvasive endometrioid tumours, focal mucinous differentiation and vascular invasion. Pathology. 2009; 41: 454-459.
- Mutch DG. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. J Gynecol Oncol. 2009; 115: 325-328.
- Nofech-Mozes S, Ackerman I, Ghorab Z, Ismiil N, Thomas G, Covens A, et al. Lymphovascular invasion is a significant predictor for distant recurrence in patients with early-stage endometrial endometrioid adenocarcinoma. American journal of clinical pathology. 2008; 129: 912-917.
- Guntupalli SR, Zighelboim I, Kizer NT, Zhang Q, Powell MA, Thaker PH, et al. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol. 2012; 124: 31-35.
- Hachisuga T, Kaku T, Fukuda K, Eguchi F, Emoto M, Kamura T, et al. The grading of lymphovascular space invasion in endometrial carcinoma. Cancer. 1999; 86: 2090-2097.
- Hertel JD, Huettner PC, Pfeifer JD. Lymphovascular space invasion in microcystic elongated and fragmented (MELF)-pattern well-differentiated endometrioid adenocarcinoma is associated with a higher rate of lymph node metastasis. International journal of gynecological pathology : Int J Gynecol Pathol. 2014; 33:127-134.
- Ismiil N, Rasty G, Ghorab Z, Nofech-Mozes S, Bernardini M, Ackerman I, et al. Adenomyosis involved by endometrial adenocarcinoma is a significant risk factor for deep myometrial invasion. Ann Diagn Pathol. 2007; 11: 252-257.
- Koshiyama M, Suzuki A, Ozawa M, Fujita K, Sakakibara A, Kawamura M, et al. Adenocarcinomas arising from uterine adenomyosis: a report of four cases. 2002; 21: 239-245.
- Abushahin N, Zhang T, Chiang S, Zhang X, Hatch K, Zheng W. Serous endometrial intraepithelial carcinoma arising in adenomyosis: a report of 5 cases. Int J Gynecol Pathol. 2011; 30: 271-281.
- Orejuela FJ, Ramondetta LM, Smith J, Brown J, Lemos LB, Li Y, et al. Estrogen and progesterone receptors and cyclooxygenase-2 expression in endometrial cancer, endometrial hyperplasia, and normal endometrium. Gynecol Oncol. 2005; 97: 483-488.
- Stewart CJ, Little L. Immunophenotypic features of MELF pattern invasion in endometrial adenocarcinoma: evidence for epithelial–mesenchymal transition. Histopathology. 2009; 55: 91-101.
- Stewart CJ, Crook ML, Lacey J, Louwen K. Cytokeratin 19 expression in normal endometrium and in low-grade endometrioid adenocarcinoma of the endometrium. Int J Gynecol Pathol. 2011; 30: 484-4891.
- Kabukcuoglu S, Oner U, Ozalp SS, Dundar E, Yalcin OT, Colak E. Prognostic significance of fascin expression in endometrioid carcinoma. Eur J Gynaecol Oncol. 2006; 27: 481-486.
- Gun BD, Bahadir B, Bektas S, Barut F, Yurdakan G, Kandemir NO, et al. Clinicopathological significance of fascin and CD44v6 expression in endometrioid carcinoma. Diagn Pathol. 2012; 7: 80.
- Stewart CJ, Crook ML, Manso L. Fascin expression in low-grade uterine endometrioid adenocarcinoma: correlation with microcystic, elongated and fragmented (MELF)-type alteration at the deep invasive margin. Histopathology. 2011; 59: 73-80.
- Saito T, Nishimura M, Yamasaki H, Kudo R. Hypermethylation in promoter region of E‐cadherin gene is associated with tumor dedifferention and myometrial invasion in endometrial carcinoma. Cancer. 2003; 97: 1002-1009.
- Yemelyanova A, Gown AM, Holmes BJ, Ronnett BM, Vang R. PAX8 expression in uterine adenocarcinomas and mesonephric proliferations. Int J Gynecol Pathol. 2014; 33: 492-499.
- Mhawech-Fauceglia P, Wang D, Samrao D, Godoy H, Pejovic T, Liu S, et al. Pair-Box (PAX8) protein-positive expression is associated with poor disease outcome in women with endometrial cancer. Br J Cancer. 2012; 107: 370-374.
- Dobrzycka B, Terlikowski SJ, Garbowicz M, Niklinski J, Chyczewski L, Kulikowski M. The prognostic significance of the immunohistochemical expression of P53 and BCL-2 in endometrial cancer. Folia Histochem Cytobiol. 2011; 49: 631-635.