Case Report
Tubo-Ovarian Abscess Misdiagnosed Ovarian Malignancy
Kong FD1, Zhao L2, Guo R1 and Zang H1*
1Department of Obstetrics and Gynecology, The First Affiliated Hospital of Dalian Medical University, China
2Department of Radiology, The First Affiliated Hospital of Dalian Medical University, China
*Corresponding author: Hong Zang, Department of Obstetrics and Gynecology, The First Affiliated Hospital of Dalian Medical University, China
Published: 15 Jul, 2016
Cite this article as: Kong FD, Zhao L, Guo R, Zang H.
Tubo-Ovarian Abscess Misdiagnosed
Ovarian Malignancy. Clin Oncol. 2016;
1: 1041.
Abstract
Tubo-ovarian abscess (TOA) is a severe sequele of pelvic inflammatory disease. The diagnosis is
dependent on the aspects of medical history, physical examination, laboratory tests and imaging.
However, the dilemma exists in differentiation of TOA and ovarian malignant tumor sometimes. We
present a case of TOA misdiagnosed as pelvic malignancy because of its untypical clinical features,
malignant features of imaging and high level of serum CA125, further discuss the importance of
medical history, physical examination and imaging for correct diagnosis of TOA.
Keywords: Tubo-ovarian abscess; Diagnosis; Imaging; CA125
Introduction
The dilemma exists sometimes in differentiation of Tubo-ovarian abscess (TOA) and ovarian malignant tumor. We present a case of TOA misdiagnosed as pelvic malignancy and discuss the importance of subtle features in medical history and role of imaging for correct diagnosis of TOA.
Case Presentation
A 37 years old woman, gravida 2, para 1, was admitted with a complaint of persistent lower
abdominal pain for one week. She started to feel lower abdominal pain, which was dull and
continuous in nature, complicated with a sensation of pelvic distention and inability of passing
flatus or feces a week before. Her abdominal pain was aggravated radiating to the back accompanied
with nausea and lack of appetite the day after. She visited the hospitals successively in Beijing
and her hometown near to Dalian, where she was suspected of having pelvic masses according to
findings from the ultrasonography and an elevated serum level of CA125 to 832.5U/L. The patient
was referred to the First Affiliated Hospital of Dalian Medical University for further diagnosis and
management. She had been feeling lassitude and lost about 10 kg of weight over the week. She
denied history of fever and had not taken any antibiotics. Her menstrual cycle was regular and last
menstrual period was 4 weeks previously with a prolonged duration of vaginal bleeding for about 20
days. Her past medical history was unremarkable.
On arrival, the patient was slightly pale, T: 37.4ºc. Her abdomen was soft with mild tenderness
in the lower quadrants, no rebound tenderness and guarding resistance. Gynecologic examination
discovered bloody discharge and mild tenderness on cervical motion. The uterus was anteverted and
slightly enlarged. Two mildly tender cystic masses around 7 and 6 cm respectively, one was located
in the right adnexal area behind the uterus, and the other was in the left above the uterus. The masses
were fixed to the uterus and pelvic side walls. Blood routine test showed white blood cell count 10.2
x 109/L with 73% neutrophils, hemoglobin 81g/dL. Transvaginal ultrasonography (TU) revealed
two cystic masses with an internal septation and papillomatous nodule. Uterus was slightly enlarged
with endometrial thickness of 15 mm. Contrast-enhanced tomography (CT) further demonstrated
two masses with hypoechogenicity, 5.82 x 8.77cm and 4.24 x 8.07cm, located in right and left
adnexal areas respectively. The masses had irregular thickened and ill-defined wall with an internal
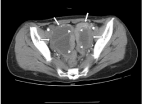
septation and mural nodule. A small amount of ascites was noted (Figure 1). Serum level of CA125
was increased to 916.7 U/mL and β-HCG was unexpectedly elevated by 110 IU/L.
After six days of antibiotic therapy with ceftriaxsone sodium IV 3g daily, abdominal pain was
persisted and temperature was fluctuated between 37~38ºc. A repeated TU reported no changes in
size of the pelvic masses. Laporatomy was performed showing hyperemic uterus, two para-uterine
masses of 8 and 6 cm both adherent to surrounding tissue, including omentum, ascending and
rectosigmoid colon, pelvic side walls and the retro-peritoneum. The adhesions were released and
grayish pus spill out from ovaries and fallopian tubes, suggesting formation of TOA. The pockets
of pus were opened and drained followed by abundant lavage. A pelvic drainage tube was retained
in cul-de-sac. A sample of pus was referred for culture and sensitivity
test and found no growth of any microbial. A biopsy of the cystic wall
confirmed fibrosis with acute inflammatory changes. The patient was
continuously put on intravenous ceftriaxsone sodium daily after the
surgery. She recovered well and was discharged home on the 7th day.
Figure 1
Figure 1
CT image displaying ovarian masses (short arrows) and structural
tubes of fallopian tubes (triangle arrows).
Discussion
TOA is a severe sequela of pelvic inflammatory disease (PID).
The diagnosis is dependent on medical history, physical examination,
laboratory tests and imaging. The present case was misdiagnosed to
malignant tumors of ovaries because of atypical history of PID and
similar presentations of the CA125 level and the imaging of masses
to ovarian malignancy.
The diagnosis of pelvic infection is most often made clinically,
based on a combination of pelvic pain, fever and leucocytosis.
TOA is one of sequelae of PID. However not all cases of TOA are
associated with classical features of fever, pelvic pain. In the case,
the patient had no pyrexia and pelvic tenderness. Therefore, TOA
should not be excluded even one or two clinical signs are absent [1].
Some factors are associated with an increased risk for development
of TOA, i.e. predisposing factors, including multi-sexual partners,
peri- menstruation /- induced abortion and low socioeconomic
status etc [2]. Taking consideration of the factors is very helpful in
making correct diagnosis of TOA. In the present case, we ignored
the evidence of a slightly elevated β-hCG level, partly because the
patient did not disclose the earlier history of medical abortion, and
the low socioeconomic status. These factors were subtle and valuable
information for the correct diagnosis.
Sometimes the infectious episode may have gone unnoticed, and
the patient presents with an undetermined pelvic mass that needs
to be characterized, where the challenge in that situation is not to
confuse it with ovarian cancer [3]. In our case, the images of masses
from TU and CT were indicative of ovarian malignancy reported
by the sonographer and radiologist at our hospital. However, it has
been reported that the image of TOA from TU may not be specific. It
may present as cystic or cystic solid or multiple internal echoes [4].
A report on imaging of TOA emphasized that CT imaging may play
an important role in the diagnosis of TOA because they observed
typical signs of imaging in 22 cases, [1] cystic/ cystic solid masses
with ill-defined walls, and no enhancement of cystic component but
enhancement of walls [2] tubular structure or sausage changes of
fallopian tubes next to pelvic masses [3] thickened cystic walls with
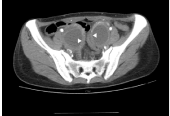
regular/smooth inner margins [5,6]. We re-reviewed the imaging
of our case and indeed found these signs (Figure 1 and 2). MRI can
be a valuable alternative to the CT examination for distinguishing a
tubo-ovarian abscess from ovarian cancer depending on T1-, T2- and
diffusion- weighted images [7].
Though CA125 is elevated in a number of benign conditions such
as endometriosis, PID and adenomyoma, however if the level is up to
1000 U/mL, the specificity for ovarian cancer reaches 99% [8]. CA125
was reached by 916.7 U/mL in the case. It is emphasized further that
the image and CA125 level may aid in a correct diagnosis but should
be combined with clinical features and other laboratory tests.
Figure 2
Figure 2
CT image displaying ovarian masses of thickened cystic walls with
regular/smooth inner margins (triangle arrows).
Conclusions
Clinical features of TOA might not be classical however subtle medical history and predisposing factors are valuable information for correct diagnosis. Furthermore, CT imaging is helpful but needed to be characterized in the diagnosis of TOA.
References
- Krivak TC, Cooksey C, Propst AM. Tubo-ovarian abscess: diagnosis, medical and surgical management. Compr Ther. 2004; 30: 93-100.
- Halperin R, Levinson O, Yaron M, Bukovsky I, Schneider D. Tubo-ovarian abscess in older women: is the women’s age a risk factor for failed response to conservative treatment? Gynecol Obstet Invest. 2003; 55: 211-215.
- Thomassin-Naggara I, Darai E, Bazot M. Gynecological pelvic infection: what is the role of imaging? Diagn Interv Imaging. 2012; 93: 491-499.
- Varras M, Polyzos D, Perouli E, Noti P, Pantazis I, Akrivis Ch. Tubo-ovarian abscesses: spectrum of sonographic findings with surgical and pathological correlations. Clin Exp Obstet Gynecol. 2003; 30: 117-121.
- Zhong LD, Feng-di, Xia Shu-mei, Zhang Jian-zhen, Yang Long-tang. The Value of CT and MR in the diagnosis of tubo-ovarian abscess. J Med Imaging. 2010; 20: 224-226.
- Ma C, Ming B, Zeng Q, Chen SJ, Tang JF, Wang Zhou X. Multi-slice spiral CT in the diagnosis tubo-ovarian abscess and hydrosalpinx. ChiIlese Journal of Medical Imaging. 2014; 22: 87-90.
- Michielsen K, Vergote I, Op de Beeck K, Amant F, Leunen K, Moerman P, et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: a clinical feasibility study in comparison to CT and FDG-PET/CT. Eur Radiol. 2014: 24: 889-901.
- Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. J Clin Pathol. 2005; 58: 308-312.