Research Article
Stereotactic Body Radiotherapy for Lymph Node Relapse in Ovarian Cancer
Trippa F*, Casale M, Draghini L, Anselmo P, Arcidiacono F and Maranzano E
Radiation Oncology Centre, S.Maria Hospital, Terni, Italy
*Corresponding author: Fabio Trippa, Radiation Oncology Centre, “S.Maria” Hospital, Italy
Published: 15 Jul, 2016
Cite this article as: Trippa F, Casale M, Draghini L,
Anselmo P, Arcidiacono F, Maranzano
E. Stereotactic Body Radiotherapy
for Lymph Node Relapse in Ovarian
Cancer. Clin Oncol. 2016; 1: 1038.
Abstract
Purpose: To evaluate the effectiveness and toxicity of stereotactic body radiotherapy (SBRT) for
lymph node relapse in patients with ovarian cancer.
Material and Methods: Between August 2008 and March 2015, 11 patients were treated with SBRT
on 20 lymph nodes of previous ovarian cancer. All patients at the time of ovarian cancer diagnosis
were submitted to surgery and at least one line of chemotherapy (range, 1-3). Median age was 64
years (range, 49-74), and primitive histology was siero-papillar and endometrioid carcinoma in 8
(72%) and 3 (28%) patients, respectively. Median time between first diagnosis and lymph node
relapse was 79 months (range, 16-171). Lymph node recurrence was documented with PET-CT
as the only site of disease. Response was evaluated with PERCIST (PET Response Criteria in Solid
Tumors) criteria.
Results: Median follow-up was 24 months, median Gross Tumor Volume (GTV) 5.35 cc (range,
1.5-18.3), median Planning Target Volume – obtained adding an isotropic margin of 5 mm to the
GTV– 15.6 cc (range, 4.2-85.7). The lymph node relapsing sites were 14 (70%) sub-diaphragmatic
(i.e., pelvic and lumbo-aortic), and 6 (30%) mediastinal. Fractionation schemes were 5 x 8Gy in
10 (50%), 5 x 7Gy in 2 (10%), 5 x 6Gy in 3 (15%) and 5 x 5Gy in 5 (25%) lymph nodes. Outcome,
evaluated with PET-CT 3 months after SBRT, showed a complete metabolic response in all treated
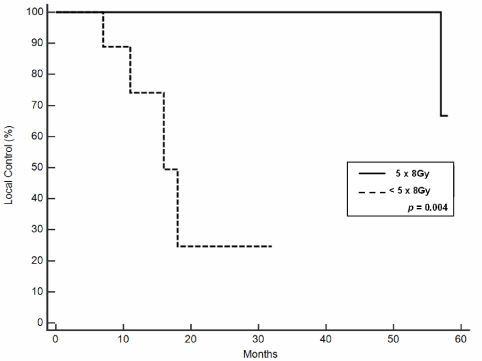
lesions. Local control (LC) at 2- and 5 years was 73% +/- 12 and 48% +/- 20, respectively and median
duration of LC was 57 months. Outcome resulted related to administered doses. In fact, at the
univariate analysis, LC was significantly better in 5 x 8Gy group versus 5 x < 8Gy group (p = 0.004).
Cancer specific survival and overall survival at 2- and 5 years were 87% +/- 11 and 73% +/- 16 and
78% +/- 14% and 65% +/- 16, respectively. We did not register acute or late toxicity after SBRT.
Conclusions: All ovarian cancer patients submitted to SBRT for lymph node relapse had a durable
complete metabolic response without toxicity. Outcome was related to higher doses. The good rates
of cancer specific and overall survivals were probably associated to an accurate patient selection that
identified true oligometastatic lesions suitable for an ablative local therapeutic approach as SBRT.
Keywords: Stereotactic body radiotherapy; Ovarian cancer; Oligometastases
Introduction
Oligometastatic disease is considered an intermediate state between localized and systemic
cancer [1]. There is rising evidence that patients with a limited number of metastases have a better
prognosis than those with extensive disease. The increasing ability to detect and cure earlier state
metastatic cancer mandates the expansion of staging and classifying metastases in the TNM system
[2] (e.g, patients with a solitary metastasis in a single organ are defined M1, whereas oligometastases,
limited to 1 organ, number < 5 and < 5 cm in total, are defined M2).
Ablation of solitary metastases (e.g, M1, M2) is in the domain of surgeon and radiation
oncologists. Stereotactic Body Radiotherapy (SBRT) is an emerging approach in the treatment
of single lesions that combines the complex dose distributions of intensity modulated radiation
therapy with the accuracy, reproducibility, and high doses of external beam radiotherapy. Dose
is usually divided in 1-5 fractions given in one week. Body immobilizers may be used to maintain
spatial relationships during treatment sessions. The precision of this technique allows delivery high
dose of radiotherapy to the tumor while sparing surrounding normal tissue. Recent published data
show that epithelial ovarian cancer recurs in 17-35% of the patients with early disease and in 30-
75% patients with advanced disease [3]. Relapses of disease have a poor prognosis and are almost
always fatal. Frequency of nodal involvement among relapses is well known, but isolated lymph
node recurrence is less described in literature [4,5]. Generally, these
patients are treated with surgery and/or chemotherapy. In our study
we evaluated the local control (LC), overall survival (OS) and toxicity
in 11 consecutive patients with previous diagnosis of ovarian cancer
who have relapsed in localized lymph node and was submitted to
SBRT.
Material and Methods
Eligibility
Patients who entered the study were enrolled from August 2008
and March 2015. Inclusion criteria were a history of ovarian cancer
and limited lymph node relapse (M1, M2) showed by diagnostic
imaging: computed tomography (CT) and 18F-FDG positron emission
tomography (PET)-CT scan.
Treatment planning
Treatment set-up was performed with a multi-slice CT, 2.5 mm
thick with 0 mm gap between scans were acquired. A stereotactic
body frame with a rigid body fixation system and a fiducially box
were used. The use of contrast was evaluated case by case. Gross
tumor volume (GTV) was defined as the radiologically visible tumor.
Clinical target volume (CTV) was coincident with GTV and planned
target volume (PTV) was designed as GTV/CTV plus an additional
isotropic margin of 5 mm. After dosimetric and physic calculations
for each treatment, the GTV/CTV was re-contoured in the image
slices obtained with a verification CT (i.e, PTV must include both
GTV/ CTV using the image fusion of the first CT and the verification
CT). Coplanar dynamic arcs were conformed around PTV, typical
field-shape margin was 2 mm, and micro-multileaves (MLC) set-up
changed every 10 degrees to follow the possible variations on target
profile through the beam eye view system. Treatment was delivered
with a 5-MV X-rays linear accelerator with external dynamic MLC.
Before each SBRT fraction, accuracy of treatment was evaluated
with daily MV portal imaging. The SBRT dose was prescribed to the
isocentre and the minimal coverage accepted dose was 90% with a
maximum dose not exceeding 110%. Five consecutive daily fractions
were delivered.
Response, toxicity evaluation and statistical analysis
Response was assessed with PET-CT using PET Response Criteria
in Solid Tumors (PERCIST 1.0) [6]. Local control was achieved
if there was a lack of progression (i.e., any metabolic response or
stable metabolic disease). All patients were evaluated with PET-CT
3 months after the end of SBRT, and at 4 months interval thereafter.
Acute toxicity was scored according to the National Cancer
Institute Common Terminology Criteria for Adverse Effects Version 4
(CTCAE v 4.0). Late toxicity was recorded according to the Radiation
Therapy Oncology Group-RTOG and European Organization for
Research and Treatment of Cancer Late Radiation Morbidity Scoring.
A software package (MedCalc 11.1 Broekstraat 52, B-9030
Mariakerke Belgium) was used for statistical analysis. Overall survival
and duration of local control were estimated for the entire population
using the Kaplan-Meier product-limit method [7].
Results
Eleven patients with 20 lymph node recurrent lesions were treated
with median follow-up of 24 months. Fractionation schemes were 5 x
8Gy in 10 (50%), 5 x 7Gy in 2 (10%), 5 x 6Gy in 3 (15%) and 5 x 5Gy
in 5 (25%) lymph nodes. Doses were prescribed considering the organ
at risk constraints, so higher doses were used in about one half of the
treated lesions. At the time of ovarian cancer diagnosis, all patients
were submitted to surgery and at least 1 line of chemotherapy (range,
1-3). Median age was 64 years (range, 49-74), primitive histology was
siero-papillar and endometrioid carcinoma in 8 (72%) and 3 (28%)
patients, respectively. Median interval time between first diagnosis
and lymph node relapse was 79 months (range, 16-171). Table 1
shows the lesion characteristics.
At PET-CT control, 3 months after the end of SBRT, a complete
metabolic response in all treated lesions was observed. Local control
at 2- and 5 years was 73% +/- 12 and 48% +/- 20, respectively
with a median duration of 57 months. Outcome resulted related
to administered doses. In fact, at the univariate analysis, LC was
significantly better in 5 x 8Gy group versus 5 x < 8Gy group (p = 0.004;
see (Table 2 and Figure 1). After SBRT we did not register in-field
relapse, 5 (45%) patients with primary sub-diaphragmatic (i.e, pelvic
and lumbo-aortic) lymph node disease had out-field progression,
one a pelvic lymph node relapse, and remaining four a peritoneal
localization. One (9%) patient with primary mediastinal lymph node
disease recurred out-filed on new mediastinal lymph node. The two
cases with nodal progression received another SBRT, while the other
four patients with peritoneal diffusion were submitted to salvage
chemotherapy. Cancer specific and overall survivals at 2- and 5 years
were 87% +/- 11 and 73% +/- 16 and 78% +/- 14 and 65% +/- 16,
respectively. We did not register acute or late toxicity after SBRT.
Table 1
Table 2
Figure 1
Figure 2A
Figure 2B
Discussion
Lymph node metastases are well known in the course of ovarian
carcinoma, with prevalence among metastatic between 50 and 70% in
clinical and necroscopic studies [8]. Isolated lymph node relapses are
rarely described, indeed, nodal metastasis usually occurs along with
the appearance of peritoneal carcinomatosis, solid organ metastasis,
such as pleura, liver or lung. Blanchard in his retrospective analysis
on 640 patients with ovarian cancer, showed a rate of 4.2% of isolated
lymph node recurrence after primary therapy [5]. Patients were
treated with surgery, chemotherapy or conformal radiotherapy with
a median OS of 26 months. In oligometastatic cancer patients, SBRT
is an emerging ablative treatment option which can be effective in
delaying disease progression especially in limited lymph node relapse.
To reduce selection biases and to identify the true oligometastatic
patients, diagnosis of oligometastasis should be carefully made using
both CT scan and PET-CT.
Using this accurate diagnostic selection in a group of 40
oligometastatic prostate cancer patients with 47 isolated lymph node
metastases, we obtained a high LC rate with SBRT (98% of LC and
no disease recurrence in 40% of cases) [9]. In ovarian cancer few
experiences of SBRT in the treatment of recurrent or non-operative
disease are reported. Higginson submitted to SBRT patients with
isolated lung metastasis, paraaortic nodes, or vaginal cuff recurrences
after primary surgery and adjuvant therapy, and with a median followup
of 11 months, reported 79% locoregional control, 43% distant
failure, and 50% overall survival [10]. Kunos included only three
cases in their experience but involved patients with multiple local and
distant recurrences treated with multiple courses of chemotherapy,
prior radiation, and/or surgeries. One patient after primary surgery
and chemotherapy was submitted to SBRT for a third relapse of
her cancer and obtained a stabilization of disease without evidence
of progression for 9 months, Another patient was free of disease at
10 months after SBRT used for a persistent vaginal lesion following
primary debulking, several chemotherapy courses, and external
pelvic radiotherapy. A third patient who underwent SBRT after
multiple treatments (i.e., surgery, chemotherapy and radiotherapy)
had stable disease at 6 months follow-up with no more than grade 2
acute toxicities. [11]. Finally, Deodato described four cases of SBRT
in ovarian cancer, 3 patients were without evidence of disease at 37,
31, and 19 months after undergoing SBRT to presacral lymph nodes,
hepatic lesions, and supraclavicular nodes, respectively. One patient
was alive with disease at 18 months after SBRT dosing to anterior
mediastinal and left internal mammary nodes [12]. So considering
the few data published in this field, our series can be relevant because
analyse the subset of ovarian cancer patients with only oligometastatic
lymph node recurrence. Outcome resulted related to administered
doses. In fact, at the univariate analysis, LC was significantly better
in 5 x 8Gy group versus 5 x < 8Gy group (p<0.004). In our analysis,
outcome seems less satisfying in patients with sub-diaphragmatic (i.e.,
pelvic and lumbo-aortic) disease because of a peritoneal progression
also in absence of in-field relapse. We observed that patients with
supra-diaphragmatic (mediastinal) lymph node localization had a
higher rate of LC respect to those with sub-diaphragmatic disease.
Sub-diaphragmatic nodal relapse in ovarian cancer can be closely
related to the synchronous presence of microscopic peritoneal
disease that cannot be evidenced by PET-CT. In fact, the 4 patients
who developed peritoneal carcinomatosis had a previous subdiaphragmatic
lymph node localization treated with SBRT. At
univariate analysis, sub- versus supra-diaphragmatic localization
did not result statistically significant probably for the relatively low
number of recruited patients. To our knowledge, this is one of the
largest experience analysing the role of SBRT in oligometastatic
ovarian cancer patients affected by isolated node metastasis. SBRT is
safe, effective, and minimally invasive in the eradication of limited
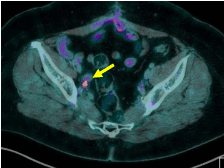
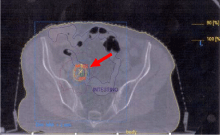
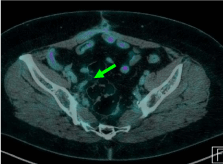
nodal metastases. At PET-CT control all patients obtained a complete
metabolic response and maintained their in-field metabolic remission
until last evaluation, furthermore we did not report acute or late
toxicity (Figure 2). Outcome was related to higher doses. The good
rates of cancer specific and overall survivals were probably associated
to an accurate patient selection that identified true oligometastatic
lesions suitable for an ablative local therapeutic approach as SBRT.
Figure 2C
References
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011; 8: 378-382.
- Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol. 2006; 16: 120-130.
- Gadducci A, Cosio S, Zola P. The clinical outcome of epithelial ovarian cancer patients with apparently isolated lymp node recurrence: A multicenter retrospective Italian study. Gynecol Oncol. 2010; 116: 358-363.
- Dvoretsky PM, Richards KA, Angel C. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988; 19: 57-63.
- Blanchard P, Plantade A, pages C. Isolated lymph node of epithelial ovarian carcinoma. Outcomes and prognostic factors. Gynecol Oncol. 2007; 104: 41-45.
- Wahl RL, Jacene H, Kasamon Y. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009; 50:122S-150S.
- Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc.1958; 53: 457-481.
- Burghardt E, Girardi F, Lahousen M. Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991; 40: 103-106.
- Ingrosso G, Trippa F, Maranzano E, Carosi A, Ponti E, Arcidiacono F, et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Uro. 2016; 0.1007/S00345-016-1860-0.
- Higginson DS, Morris DE, Jones EL, Clarke-Pearson D, Varia MA. Stereotactic body radiotherapy (SBRT): Technological innovation and application in gynecologic oncology. Gynecol Oncol. 2011; 120: 404-412.
- Kunos C, Chen W, DeBernardo R, Waggoner S, Brindle J, Zhang Y, et al. Stereotactic body radiosurgeryfor pelvic relapse of gynecologic malignancies. Technol Cancer Res Treat. 2009; 8: 393-400.
- Deodato F, Macchia G, Grimaldi L, Ferrandina G, Lorusso D, Salutari V, et al. Stereotactic radiotherapy in recurrent gynecological cancer: a case series. Oncol Rep. 2009; 22: 415-419.