Case Report
Case Report of Small Hepatocellular Carcinoma Complicated with an Isolated Portal Vein Tumor Thrombosis
Shi JY1, Wang XY1, Zhou J1,2, Fan J1,2 and Gao Q1*
1Institute of Liver Cancer, Fudan University, China
2Institute of Biomedical Sciences, Fudan University, China
*Corresponding author: Qiang Gao, Liver Cancer Institute, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, P.R. China
Published: 18 Jul, 2016
Cite this article as: Shi JY, Wang XY, Zhou J, Fan J, Gao
Q. Case Report of Small Hepatocellular
Carcinoma Complicated with an
Isolated Portal Vein Tumor Thrombosis.
Clin Oncol. 2016; 1: 1030.
Abstract
HCC has a high propensity to invade into the portal vein by direct venous extension or metastasis,
which may cause tumor thrombosis that occurs in up to 70% of patients. It is well recognized that
Portal Vein Tumor Thrombosis (PVTT) is a major detrimental prognosticator in HCC and almost
all staging or prognostic systems for HCC include PVTT as an important parameter. Herein, we
reported an interesting HCC case, that is, a male patient with a 30-mm mass in the right lobe and an
isolated “mural” portal vein thrombosis in the right portal branch. The patient received hepatectomy
and removal of the portal vein thrombosis. The pathological diagnosis proved that both the liver
mass and the portal vein thrombosis were HCC. According to our medical records, it's a rare case of
small hepatocellular carcinoma with such severe PVTT.
Conclusion: It is vital to pay more attention on PVTT in HCC patients for evaluating the
postoperative outcome and optimizing treatment strategies.
Keywords: Portal vein tumor thrombosis; Small hepatocellular carcinoma
Introduction
HCC is the second most common cause of cancer-related death worldwide and accounts for more than 600,000 new cases per year [1]. Only ~30% of patients with HCC are eligible for curative treatment involving liver transplantation and surgical resection [2]. Of note, HCC has a propensity to invade into the portal vein by direct venous extension or metastasis, which may cause tumor thrombosis that occurs in up to 70% of patients [3,4]. Portal Vein Tumor Thrombosis (PVTT) may lead to wide intrahepatic dissemination of the tumor, exacerbate portal hypertension, and reduce portal flow, resulting in upper gastrointestinal hemorrhage and deterioration of liver function. Previous studies have reported that the median survival of patients with PVTT was 2.7-4.0 months if left untreated, indicating that PVTT is a major detrimental prognosticator in HCC [3]. Almost all staging or prognostic systems, such as the TNM, Cancer of the Liver Italian Program (CLIP), Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire (GRETCH), Japan Integrated Staging (JIS) and Barcelona Clinic Liver Cancer (BCLC), include PVTT as an important parameter [5]. Moreover, patients with PVTT at different locations in the portal vein have different prognoses after liver resection, promoting the formulation of several specific PVTT microscopic or macroscopic classifications based on the extent of the tumor thrombus in the portal vein [6,7]. Here, we presented an interesting HCC case, that is, a male patient with a small mass in the right lobe and an isolated “mural” portal vein thrombosis in the right portal branch to underline the significance of PVTT in management of HCC.
Case Presentation
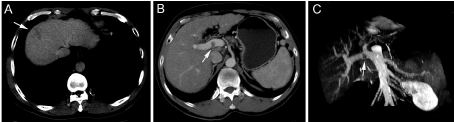
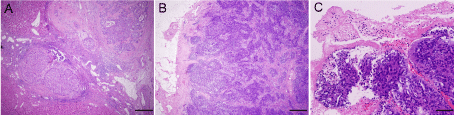
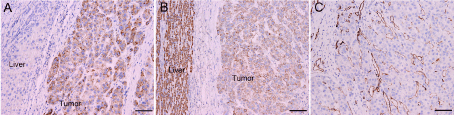
A 54-year-old man suspected with hepatocellular carcinoma (HCC) identified by surveillance with α-fetoprotein and ultrasound was admitted to our hospital. He has a history of hepatitis-B virus (HBV) positivity for over 20 years without any anti-HBV treatment; however, the development and exact duration of HBV-related cirrhosis in this patient was unclear. He denied familial history of HBV and HCC, nor any alcohol consumption. The serum α-fetoprotein was 8246.0 ng/mL (normal range: <20 ng/mL) and liver function test was normal. The patient’s Eastern Cooperative Oncology Group performance status was 0 and Child-Pugh classification was A. Contrast-enhanced computed tomography (CT) showed a 30-mm hepatic mass with an irregular margin at segment VII that appears as a hypo-attenuating lesion during venous phase (Figure 1A) with limited enhancement during arterial phase (not shown). In particular, an isolated portal vein thrombosis was observed in the right portal branch with slight enhancement (Figure 1B). The portal vein thrombosis displayed the pattern of a mural thrombosis, leaving the portal flow unaffected (Figure 1C). No definable extrahepatic metastases to the regional lymph node, lung, brain and bone were detected, as proved by multiple radiological examinations. The patient underwent segmentectomy of segment VII, removal of the portal vein thrombosis and dissection of hilar lymph nodes on Aug. 8, 2012. After the removal of the portal vein thrombosis, we performed a bloodletting with 400ml blood via portal vein to prevent tumor cells remaining. The pathology reported a moderately differentiated HCC (Edmondson grade II), with surrounding microscopic spreading nodules and a free resection margin (Figure 2A). Notably, the portal vein thrombosis was also proved to be HCC (Figure 2B and 2C). Immunohistochemical staining of the tumor showed GPC-3 (Figure 3A), HepPar-1 (Figure 3B) and CD34 (Figure 3C) positivity. The nontumor cirrhotic liver was graded as Scheuer system G2S4. No metastases were detected in the dissected lymph nodes. The patient recovered uneventfully and was discharged home on postoperative day 7. After three weeks of surgery, he got an ultrasound examination which showed a new thrombus in the right portal branch and then he received transhepatic arterial chemotherapy and embolization after one month of surgery with a mixture of 1g 5-FU, 150mg Oxaliplatin, 30mg Epirubicin and 10ml iodized oil. Unfortunately, he got severe malnutrition with ascites after discharge and died of dyscrasia on May 15, 2013.
Figure 1
Figure 1
Contrast-enhanced CT scan of hepatic mass and portal vein tumor thrombosis. (A) The hepatic mass at segment VII was indicated by an arrow. (B) The
mural thrombosis in the right portal branch was pointed by an arrow. (C) The portal vein thrombosis was shown in the coronal view.
Figure 2
Figure 2
Hematoxylin and eosin staining of the hepatic mass and portal vein thrombosis. (A) The hepatic mass was proved to be HCC with microscopic spreading
nodules. Magnification, ×40. (B and C) The cluster of carcinoma cells were observed in the portal vein thrombosis. Magnification, ×40, ×100.
Figure 3
Figure 3
Immunohistochemical staining of HCC-related markers in the tumor tissues. (A) GPC-3, (B) HepPar-1 and (C) CD34. Magnification, ×100.
Discussion
It is well known that the advent of PVTT is significantly associated
with the presence of highly metastatic HCC nodule(s) or heavy tumor
burden in the liver parenchyma. Only two previous publications
have reported HCC presenting only as PVTT with no demonstrable
tumor in the liver parenchyma outside the portal vein [8,9]. In HCC,
while the hepatic artery is the feeding vessel, the portal vein serves
mainly as an efferent vessel. As such, the main mechanism for the
formation of PVTT is that tumor cells invade efferent vessels, engorge
in the vascular cavity, and extend beyond the capsule to branches of
the portal vein [4]. Hence, PVTT usually presents as a continuous
architecture that starts microscopically from the HCC nodule,
prorogate along portal branches and ends with occlusive thrombus
formation. To our knowledge, this case represents the first established
small HCC with isolated PVTT, in which the PVTT showed the
pattern of mural thrombosis, leaving the portal flow unaffected. The
exact mechanism for this distinct type of "partial or patent" PVTT
remains largely unknown. A group of risk factors, including the
location of the PVTT near the divaricator of portal trunk where
turbulence of blood flow is likely to occur, portal hypertension due
to liver cirrhosis, communication between hepatic artery and portal
vein, and nourishing vessels alone the portal vein, may collaborately
have important roles.
The treatment of HCC with PVTT is complicated and
controversial. Accumulated evidence has shown that certain
therapeutic modalities, including surgery, radiotherapy, transcatheter
arterial chemoembolization, hepatic arterial infusion chemotherapy,
and combined treatment can improve the survival of HCC patients
with PVTT [10]. With improvement of surgical techniques, more
and more patients with HCC and PVTT benefit from surgical
intervention such as liver resection with PVTT removal. In Japan
and western countries, only a small proportion of HCC patients
with PVTT undergo hepatic resection. In China, more than 30%
of patients are found to have advanced HCC with PVTT when the
liver cancer is first diagnosed, among which about 10% receive liver
resection [11]. We chose surgical resection and PVTT removal for this
case, considering the fact that only single HCC nodule was detected
in a liver with preserved function. In addition, before operation and
pathological diagnosis, we cannot rule out the possibility that the
isolated thrombosis in this case may be a benign one, although slight
enhancement of the thrombus was seen during arterial and venous
phase of CT scan.
In conclusion, the presence of "partial or patent" PVTT in
untreated HCC patients, especially in those with a small tumor, is
rare and not reported previously. It has been documented that tumor
vascular invasion was observed in 48.4% of HCC tumors <2.0 cm,
in 59.1% of those 2.1 to 3.0 cm, and in 73.3% of those 3.1 to 5.0 cm
in diameter [4], suggesting the possibility of development of PVTT
during very early stage of HCC. Consequently, it is vital to consider
this factor in evaluating the postoperative outcome and in stratifying
patients for adjuvant therapies, and to develop more effective
treatment strategies for HCC with PVTT.
References
- Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61: 69-90.
- Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/ advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010; 15: 42-52.
- Lau WY, Lai EC, Yu SC. Management of portal vein tumor thrombus. In: Lau WY, editor. Hepatocellular carcinoma. Singapore: World Scientific Publishing. 2008; 739–760.
- Toyosaka A, Okamoto E, Mitsunobu M, Oriyama T, Nakao N, Miura K. Pathologic and radiographic studies of intrahepatic metastasis in hepatocellular carcinoma; the role of efferent vessels. HPB Surg. 1996; 10: 97-103.
- Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist. 2010; 15: 23-33.
- Fujita N, Aishima S, Iguchi T, Mano Y, Taketomi A, Shirabe K, et al. Histologic classification of microscopic portal venous invasion to predict prognosis in hepatocellular carcinoma. Hum Pathol. 2011; 42: 1531-1538.
- Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007; 54: 499-502.
- Lim JH, Auh YH. Hepatocellular carcinoma presenting only as portal venous tumor thrombosis: CT demonstration. J Comput Assist Tomogr. 1992; 16: 103-106.
- Poddar N, Avezbakiyev B, He Z, Jiang M, Gohari A, Wang JC. Hepatocellular carcinoma presenting as an incidental isolated malignant portal vein thrombosis. J Gastrointest Cancer. 2012; 43: 486-489.
- Jia Fan, Jian Zhou, Zhi-Quan Wu, Shuang-Jian Qiu, Xiao-Ying Wang, Ying-Hong Shi, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2005; 11: 1215-1219.
- Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011; 18: 74-80.