Review Article
Breast Cancer Screening and Treatment in Resource Limited Environments: Challenges and Solutions
Duda RB*
Division of Surgical Oncology, Beth Israel Deaconess Medical Center, USA
*Corresponding author: Rosemary B. Duda, Department of Surgery, Harvard Medical School, Division of Surgical Oncology, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215, USA
Published: 29 Jul, 2016
Cite this article as: Duda RB. Breast Cancer Screening
and Treatment in Resource Limited
Environments: Challenges and
Solutions. Clin Oncol. 2016; 1: 1029.
Abstract
Breast cancer is a common health problem for women worldwide and is the leading cause of cancer mortality globally. Screening measures can be instituted even if modern technology in the form of mammography or ultrasound does not exist. However, there are multiple challenges to screening for breast cancer in resource-limited environments, including underestimation of the risk of breast cancer in these populations, time constraints on physicians, and a cultural aversion to a mastectomy in many communities. Proposed solutions include culturally sensitive public health initiatives that serve to educate women and professional and government personnel about the role of screening for breast cancer as well as the treatment options for each specific target population. Other strategies to assist with screening include teaching nurses and community health workers to perform clinical breast examinations and focused epidemiologic research for pertinent population risk factors, incidence rate and prevalence rate.
Introduction
Breast cancer is a relatively common health problem for women worldwide. It has been estimated
by the International Agency for Research on Cancer (IARC) and World Health Organization
(WHO) that 1.7 million women worldwide had been diagnosed with breast cancer in 2012 (Figure
1) [1-3]. In addition, the risk of breast cancer increases with age, and as diseases that previously led
to premature death are treated, including infections, malaria and childhood illnesses for example,
more women are at risk for breast and other cancers [4].
Screening for cancer is performed to detect cancers that are not clinically evident and implies
that a treatment option is available for those identified with a cancer [5]. Screening also implies
that cancers will be found at an early stage with a better prognosis in comparison to cancers that
present clinically at an advanced stage. Screening guidelines do change with time, and must reflect
the benefit for screening of the population of interest versus any harm that may result [6].
One ethical dilemma exists when screening to detect early stage breast cancer in resource-limited
environments (RLE). While breast conservation management is the standard of care for women in
developed countries diagnosed with early stage of disease, it is largely an unavailable treatment
option for women in isolated and resource limited countries that may lack surgical expertise and
access to radiation therapy and systemic therapy. While the standard of care once was a mastectomy
for every woman diagnosed with breast cancer, it is not a culturally acceptable option for some
women living in some of the less developed areas of the world.
Challenges and Proposed Solutions
There are many challenges to screening for breast cancer in RLE. There are also challenges and
limitations to treating women with breast cancer once detected.
The following challenges and solutions are offered for thought, reflection and discussion based
upon the author’s experience in working in several resource-limited countries.
A. Challenge: Perception of risk by professionals and general public
It had been a previous misperception that women who lived in rural RLE were not at a substantial
risk of breast cancer. The risk factors for breast cancer as we know them seem not to fit the risks of
women who reside in RLE, such as nulliparity, lack of breast feeding, and late age of first full-term
pregnancy [7]. However, we have found that women are developing breast cancer at an age younger
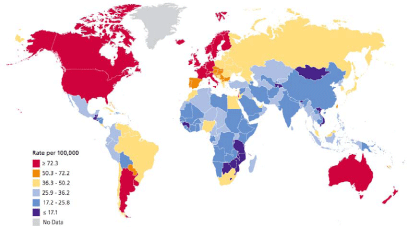
than anticipated in RLE (Figure 2), and who do not have the classic risk factors [4]. In addition,
mortality from breast cancer is the highest of any cancer worldwide [8].
A small number of women diagnosed with breast cancer in a
community may not alert the stakeholders that breast cancer is a
serious health concern for the area. For example, in 2014, six women
were newly diagnosed with breast cancer in Yap, Federated States of
Micronesia, a remote group of islands in the western Pacific Ocean.
Based upon the 2010 census data for women in Yap State, which has a
total population of approximately 11,000, it is estimated that that the
age adjusted incidence rate of breast cancer is 234 per 100,000 women
[9,10]. This incidence rate exceeds the age adjusted incidence rate of
122.2 per 100,000 women in the United State [11].
B. Proposed solution: Research and education
Epidemiologic research into the risk factors for breast cancer in
RLE as well as the true incidence and prevalence of disease would
help government’s direct resources into early detection, treatment
and ultimately prevention of breast cancer.
Education of both professionals and the general public is also
a key component to allocating resources appropriately. Physicians
should be aware of the incidence of breast cancer and include a
clinical breast examination (CBE) annually for all women. In areas
of major time constraints when seeing patients in the outpatient
setting, physician extenders (nurses, community health workers) can
be trained to perform a CBE and refer to the physician abnormal and/
or suspicious findings.
As a component of education, a public health awareness
campaign can be initiated at the local ground level to encourage
women to perform a self-breast examination (SBE) and to request an
examination of the doctor on an annual basis and not just when the
woman detects an abnormality.
To test the hypothesis that education can impact on health
awareness and practice, a knowledge, attitude and practice (KAP)
survey was conducted in rural Nicaragua. The breast health literacy
score increased from a baseline of 63% to 93% after the education
intervention [12]. Prior to the educational component of the survey,
only 16 or 198 (8.1%) had a clinical breast examination and only four
were physician initiated. Seventy-four percent of the participants
had knowledge of breast cancer. Ten women (5.1%) had previously
performed a SBE but with no clear pattern of a routine practice.
Women with a FH were five times more likely to have performed
a SBE, compared with women without a know FH (OR=5.5, 95%
CI=1.10, 27.81, p=0.037).
Participants expressed an interest in learning how to perform
a SBE. The majority of women (97.4%) stated that they were
comfortable performing a self-breast examination, 94% agreed to
perform it monthly, and 77.2% had taught a friend or family member
the technique before the 2-week follow-up assessment. In addition,
a trained community health assistant performed a CBE, with 10
abnormal examinations identified in this group of participating
women. This study showed that women with little formal education
can be successfully taught about the importance of breast health and
can successfully grasp the technique of a SBE.
A. Challenge: Time constraints on physicians
Anecdotally, many physicians do not routinely perform a clinical
breast examination (CBE) due to time constraints and large patient
volumes. This has been the author’s experience in several outpatient
settings in various urban and rural clinics. An ideal time to conduct
a CBE would be when women are seen for cervical cancer screening.
However, the queue and patient wait can be very long. An additional
five minutes per patient was frequently viewed as impractical when
you are seeing over 100 patients per day.
B. Proposed solution: Physician extenders
A CBE can be performed by trained professional assistants,
including nurses and community health workers, as demonstrated
by the KAP study in Nicaragua [12]. The trained professional can be
taught to screen for abnormalities that require a referral for physician
assessment and possible further evaluation. This allows physicians to
use their time more effectively by treating women with serious breast
health problems. The physician extenders can also be trained to teach
women to perform a self-breast examination (SBE), thus assisting
in the screening process and increasing a public health awareness of
breast health.
A. Challenge: Mammographic screening
Radiographic limitations include machines and supplies, such as
film and chemicals. Many outpatient facilities in RLE cannot afford
the equipment, and even if donated, there is maintenance required
to keep the machines operational. Disposable supplies, such as film,
must be replenished, all of which are costly. If a suspicious lesion is
found on mammography, there must be the capability of a localizing
the lesion for a biopsy and there must be the capability for treatment.
B. Proposed solution: Ultrasound evaluations, regional
mammographic machines and alternate screening in rural
communities
Many outpatient facilities do have an ultrasound for obstetrical
use. Physicians can be trained to use ultrasound to assess if a breast
mass is solid or cystic. An ultrasound can also provide characteristics
that are consistent with malignancy in the absence of a histologic
confirmation.
In countries with regional hospitals, mammography can be
added as an important health service provided for both screening
and diagnostic purposes. To eliminate the need for film, digital
radiographic machines should be considered as a practical alternative
to film. For example, in Yap, all radiographs are performed by digital
equipment and the images viewed on computers in the outpatient
clinic, which has greatly enhanced health care and circumvented the
need for film and chemicals. To date, mammograms are not available.
A SBE, combined with a CBE, may provide for a reasonable
screening approach for breast cancer in resource limited areas that
cannot offer mammography [13-18]. While these procedures are not
recommended as the sole screening methods in countries that can
provide mammographic screening, they do have a role in the absence
of mammographic screening. The Breast Health Global Initiative
(BHGI) provides screening guidelines for RLE that are appropriate
until resources become available for mammographic screening
[19,20]. The approach to breast cancer screening in RLE includes
the promotion of basic breast health knowledge, training in SBE
performance, and encouragement of CBE promotion.
A. Challenge: Accepting a mastectomy as the treatment
Fear is a great motivator to avoid breast cancer screening. Some
women may feel they would prefer not to know if they have breast
cancer in order to avoid the consequences that include an operation
as seemingly unacceptable as a mastectomy. In an environment
that does not offer radiation therapy, the safest treatment option is
a mastectomy [21]. In many areas of the world, cultural attitudes
can limit women’s acceptance of a mastectomy as a treatment
[22–24]. And in RLE, breast conservation is rarely an ideal option
either because the cancers are too large at the time of presentation
or appropriate treatment options do not exist, such as neo-adjuvant
chemotherapy to decrease the size of the cancer to make the patient
eligible for a partial mastectomy, and radiation is not available for
post breast conservation surgical procedures.
B. Proposed solution: Public health awareness campaigns,
culturally appropriate strategies, supportive counseling
Sensitivity to the culture and beliefs specific to the community
is an essential component to a successful screening and treatment
program. And it is essential to address the treatment plan that will
most likely be offered in order to appropriately prepare a woman
in advance of a diagnosis. Not only can it be a tremendous shock to
someone to learn that they have breast cancer, but the concept of a
mastectomy may cause her to refuse treatment and not return for
medical care. For example, if a woman would like to consult with a
traditional practitioner or healer rather than undergo a mastectomy,
keep the door open to provide medical care should the woman decide
to return. Work with her and perhaps even the traditional healer on
developing an acceptable approach where the woman may undergo
the necessary mastectomy and still utilize traditional therapy.
While it is difficult for many women, regardless of geographic
location to accept a mastectomy, many women do so and remain
active participants in their communities. Women who have
undergone the procedure can serve as role models and counselors
for women newly diagnosed in their communities. Physicians, nurses
and community health workers can make special efforts to explain
body image perceptions. While breast reconstruction is an unlikely
option for most women in these environments, mastectomy bras
can be locally modified and used with substitute breast prosthesis.
Counseling can help a woman adapt to the diagnosis and mastectomy
and effectively live a full and meaningful life in their community.
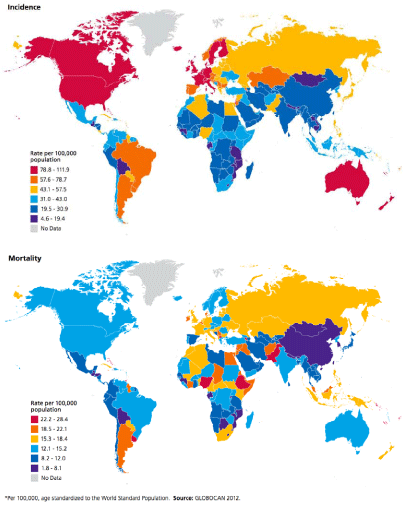
Figure 1
Figure 1
International variation in female breast cancer mortality rates, 2012.
Source: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-044738.pdf
Figure 2
Figure 2
International variation in age standardized breast cancer incidence rates, 2008.
Source: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-027766.pdf
Conclusion
There are certainly other issues that limit breast cancer screening detection and treatment, such as a lack of pathology and radiation therapy and chemotherapy. Diagnosis and treatment of a cancer is complex, frequently requiring sound clinical and surgical judgment when no pathologic assessment is available. The surgeon must be able to determine on the basis of the clinical examination whether or not a breast mass is malignant in order to make the correct treatment decisions. A public health initiative in the communities should be part of the overall health plan to inform the public about the risks of breast cancer, its presentation, and the role of screening to detect small cancers to afford a more favorable prognosis, and the treatment options locally and regionally available. These initiatives should be performed in a culturally sensitive manner appropriate for the target communities.
References
- International Agency for Research on Cancer (IARC) and World Health Organization (WHO). GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2016.
- Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJL, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011; 378: 1461-1484.
- Komen SG. The Who, What, Where, When and Sometimes Why. 2016.
- American Cancer Society. Global Cancer Facts & Figures 2nd Edition. Atlanta: American Cancer Society. 2011.
- Simon S. Why we screen for some cancers and not others. American Cancer Society. Prevention and Early Detection. 2016.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. 2016.
- Center for Disease Control. What are the risk factors for breast cancer?
- Globocan. International Agency for Cancer Research. Breast Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. World-wide Age Standardized Incidence and Mortality Rates for Women. 2016.
- Division of Statistics. 2010 FSM census data. Office of Statistics, Budget and Economic Development, Overseas Development and Compact Management.
- Duda RB. Creating a breast cancer program in Yap. Presentation at Department of Health Services, Yap State Hospital. Data based upon 2014 Yap Cancer Registry. 2015.
- Center for Disease Control. United States Cancer Statistics (USCS). 2016.
- Duda RB, Bhushan D. Teaching rural women in Nicaragua the principles of breast health. J Cancer Educ. 2011; 26: 560–565.
- Weiss NS. Breast cancer mortality in relation to clinical breast examination and breast self-examination. Breast J. 2003; 9: S86-S89.
- Bullock K, McGraw SA. A community capacity- enhancement approach to breast and cervical cancer screening among older women of color. Health Soc Work. 2006; 31: 16-25.
- Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009; 151; 727-737.
- Baxter N. Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: should women be routinely taught breast selfexamination to screen for breast cancer? CMAJ. 2001; 164: 1837-1846.
- Vahabi M. Breast cancer screening methods: a review of the evidence. Health Care Women Int. 2003; 24: 773-793.
- Anderson BO, Jakesz R. Breast cancer issues in developing countries: an overview of the breast health global initiative. World J Surg. 2008; 32: 2578-2585.
- Yip CH, Anderson BO. The Breast Health Global Initiative: clinical practice guidelines for management of breast cancer in low- and middleincome countries. Expert Rev Anticancer Ther. 2007; 7: 1095-1104.
- The Breast Health Global Initiative. 2016.
- National Comprehensive Cancer Network. NCCN Guidelines for treatment of breast cancer. 2016.
- Simon CE. Breast cancer screening: cultural beliefs and diverse populations. Health Soc Work. 2006; 31: 36-43.
- Wu TY, West B, Chen YW, Hergert C. Health beliefs and practices related to breast cancer screening in Filipino, Chinese and Asian-Indian women. Cancer Detect Prev. 2006; 30: 58-66.
- Watts T, Merrell J, Murphy F, Williams A. Breast health information needs of women from minority ethnic groups. J Adv Nurs. 2004; 47: 526-535.