Research Article
Multiple Liver Metastases from Carcinoma of the Thymus Treated with Yttrium-90 Radioembolization (Glass Microspheres): Clinical Dosimetry
Ferreira P1*, Parafita R1,3, Canudo A1,3, Oliveira C1, Rosa L1, Girão P2 and Costa DC1
1Champalimaud Centre for the Unknown, Champalimaud Foundation, Portugal
2Instituto Superior Técnico - Universidade de Lisboa, Lisboa, Portugal
3Mercurius Health, Portugal
*Corresponding author: Paulo Ferreira, Champalimaud Centre
for the Unknown, Champalimaud
Foundation, Portugal
Published: 10 Jun 2016
Cite this article as: Ferreira P, Parafita R, Canudo A,
Oliveira C, Rosa L, Girão P, et al.
Multiple Liver Metastases from
Carcinoma of the Thymus Treated with
Yttrium-90 Radioembolization (Glass
Microspheres): Clinical Dosimetry. Clin
Oncol. 2016; 1: 1024.
Abstract
Liver radioembolization with Yttrium-90 microspheres has been recognized as an emerging
treatment strategy for patients with primary hepatic malignancies and metastatic liver disease.
Furthermore, a shift toward the curative setting is desirable although Yttrium-90 radioembolization
is primarily performed in the palliative setting.
Unfortunately, there is little information on complex patient dosimetry based on real absorbed
dose distribution to the normal liver, tumor and extrahepatic tissues, from patients submitted to
radioembolization.
We herein describe the case report of our first patient (multiple diffuse liver metastases failing to
respond to sunitinib) submitted to radioembolization with Yttrium-90 glass microspheres at the
Champalimaud Centre for the Unknown (CCU), Champalimaud Foundation (FC), focusing on
complex patient clinical dosimetry based on absorbed dose calculation Stratos® algorithm software.
This new research approach enabled to calculate the tumor liver dose to be 118 Gy very close to the
pre-therapy prescribed 120 Gy. The maximum recommended normal liver dose was not reached. It
was equal to 12 Gy.
Keywords: Yttrium-90; 99mTc-MAA; Radioembolization; Dosimetry; Treatment planning;
Dose-Volume Histogram; SPECT; PET; PET/CT
Introduction
A retrospective study is underway at the CCU, FC to investigate the delivered absorbed
doses after liver Radioembolization (RE) with glass microspheres labeled with Yttrium-90 (Y90),
commercially named Therasphere®. So far, eleven patients with multiple liver metastases have been
submitted to Y90 liver RE. Quantification of absorbed doses from Positron Emission Tomography–
Computed Tomography (PET/CT) images are still a matter of debate.
Herein we describe the case of a 61 years old woman, the first CCU, FC patient submitted to Y90
liver RE in January 2015. Sixteen months after the procedure, this patient is clinically well, with no
evidence of metabolically active disease since January 2016.
This patient had been previously diagnosed with multifocal liver metastases from carcinoma
of the thymus (c-kit positive). Past therapies included imatinib (good response) and sunitinib
(initial good response and some metabolic progression thereafter). After multidisciplinary meeting
discussion and based on available literature [1], she was referred for Y90 liver RE. The patient is still
under imatinib after developing side effects from sunitinib.
Materials and Methods
In January 2015 diagnostic angiography was undertaken, with the intent to study liver arterial
blood supply (Figure 1). During this session 274 MBq of 99mTc-MAA (macroaggregates of albumin)
were injected via the main hepatic artery.
Whole body scanning and planar images of the chest and abdomen were acquired in order to
calculate the percentage of possible hepato-pulmonary shunt, as requested in preparation for the RE
treatment (Figure 2). This was followed by single photon emission computed tomography (SPECT)
to obtain a tomographic map of the 99mTc-MAA distribution within
the liver parenchyma that is directly proportional to the intrahepatic
regional arterial blood supply.
Both anterior and posterior planar images were used for the
calculation of the geometric mean counts in each region of interest
(ROI). The lung shunt value was then calculated based on the
geometric mean of the lung counts relative to the sum of the geometric
mean values of the lung and liver. The lung shunt was calculated as
3% and no other abnormal distribution of 99mTc-MAA, mainly in the
abdomen outside the liver was demonstrated. The left diaphragmatic
artery territory previously embolized with a coil during arteriography
showed no distribution of the 99mTc-MAA.
Since there is scarce and poorly characterized information
on complex dosimetry for accurate activity estimation [2-5], the
prescribed Y90 activity was calculated using the classic MIRD
formula [6], corrected for the lung shunt fraction (percentage of
hepato-pulmonary shunt) and for recommended residual activity in
the waste residue (i.e. the Y90 injector circuit after the RE procedure
was terminated):
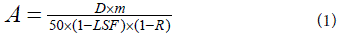
where A is the Y90 calculated vial activity of 2.906 GBq (gigabecquerel),
D is the prescribed liver dose of 120 Gy (gray), m is the
liver mass of 1.163 kg (kilograms) for 1129 cm3 (cubic centimeter)
liver volume and 1.03 g.cm-3 of mean density of the liver tissue, LSF is
the 0.03 (3%) lung shunt fraction and R is a recommended constant
value of 0.01 (1%), that represents, according to the manufacturer, the
residual activity not injected in patients and measured in the waste
residue.
The Y90 labeled glass microspheres (Therasphere®) were
administered according to current standard guidelines two weeks
later [7]. This was carried out via injecting the microspheres into
the main hepatic artery lumen to treat both lobes of the liver. No
complications or side effects were recorded during and immediately
after the procedure.
Although Y90 is considered a pure electron (β–) emitter (2.28
MeV; 99.98%), its decay cascade includes a minor β– branch (518
keV; 0.017%) to the first excited state of stable Zirconium-90 (Zr90)
at 1.76 MeV. This decays by gamma photon emission, originating
a positron–electron (β+/β–) pair production emission. It is this
emission that is used to assess Y90 biodistribution by PET imaging
[8].
Thus, after Y90 RE liver injection, PET/CT images were acquired
to obtain the Y90 distribution map within the liver parenchyma
for co-registration with previously acquired SPECT images from
the 99mTc-MAA (Figure 3). The Y90 distribution map was also later
used for patient dosimetry calculations using the Stratos® dosimetry
calculation software (not for clinical use), a module of the Imalytics
(Philips®) research workspace [9]. This dose calculation software is
enabling us to create our own “treatment planning software” (TPS),
under evaluation for subsequent implementation.
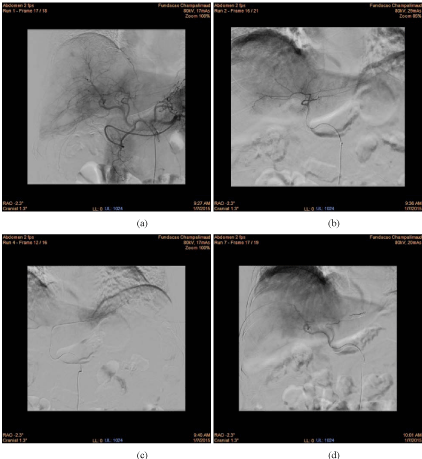
Figure 1
Figure 1
Images from the perfusion scanning diagnostic angiography of our first patient treated with Y90 RE in the liver: (a) initial arterial vasculature of the liver, (b) flow shift into the diaphragmatic artery coming from hepatic branch for segment III, precluding treatment given the risk of extra hepatic migration of Y90
microspheres and thus imposing coil embolization, (c) catheter position set for coil embolization, (d) final arterial vasculature control by main hepatic artery after
coil embolization, before the injection of the 99mTc-MAA via the main hepatic artery.
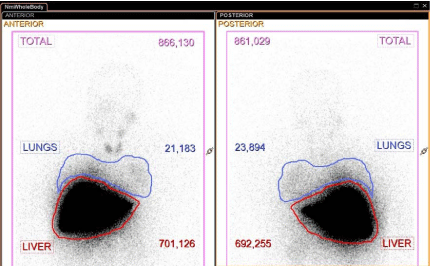
Figure 2
Figure 2
Whole body anterior and posterior images after administration of 99mTc-MAA with measurements (counts) within the regions of interest for lungs and liver used to calculate the percentage of hepato-pulmonary shunt (3% in this case).
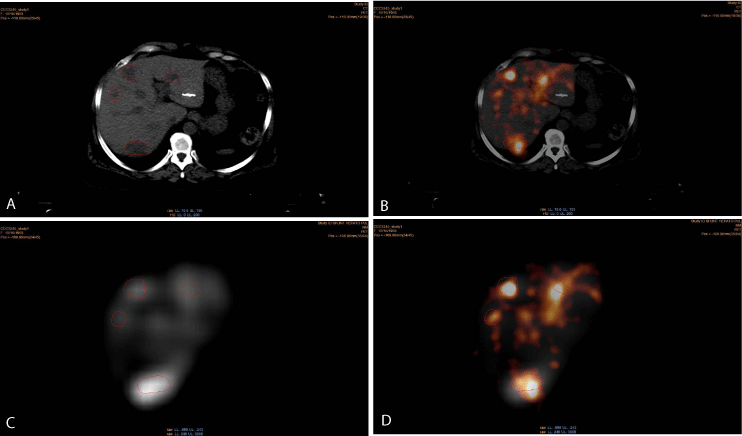
Figure 3
Figure 3
Multi-modal images (same axial slice): (a) CT image with four identifiable metastases, (b) Y90 PET/CT axial image, (c) 99mTc-MAA SPECT axial image, (d) co-registration of both Y90 (PET) and 99mTc-MAA (SPECT) images. It is important to note the higher intensity of uptake for both 99mTc-MAA and Y90
microspheres corresponding to the region of the metastatic sites that appear relatively well co-registered for all three components, i.e. morphology (CT), diagnostic
perfusion (MAA) and treatment (Y90-microspheres) maps.
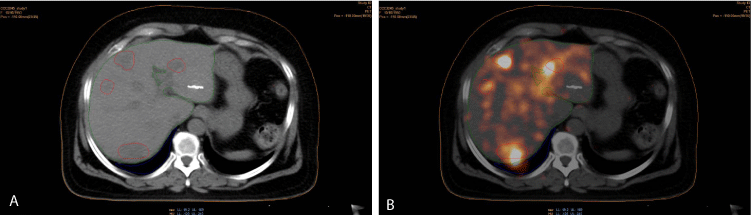
Figure 4
Figure 4
VOI contours (body/orange, liver/green, right lung/blue and tumor/red) of the patient: (a) in CT images of the Y90 PET/CT series, (b) in Y90 PET/CT
images.
Results and Discussion
When using a TPS, the main challenge is to ensure proper
calibration of the PET and SPECT images in terms of the activity
measured in Becquerel per voxel intensity.
Taking into account that 1% of the Y90 calculated vial activity
remained in the injector system as residue the activity administered
to the patient was 2.877 GBq. This body activity enabled the use of
an iterative method within the TPS, starting with a calibration factor
for PET images equal to 1 Bq/intensity, to verify the value of the
activity detected in the body. Before this calculation, readjustment
and confirmation of the CT and PET images co-registration was
performed (Figure 3B) using the manual registration tools in the TPS.
Then the segmentation tools in the TPS helped with drawing (Figure
4) all VOI contours (body, total liver, tumor, normal liver, right lung).
Finally, the dosimetry volume for absorbed dose calculation
was defined in the axial, coronal and sagittal planes. Based on this
dosimetry volume and Y90 injected activity (2.877 GBq) the absorbed
dose was calculated. Consequently, a 3D absorbed dose map was
generated with the help of TPS (Figure 5).
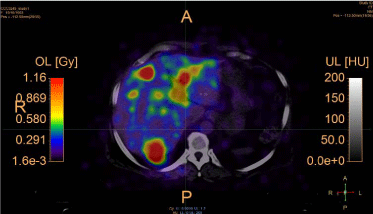
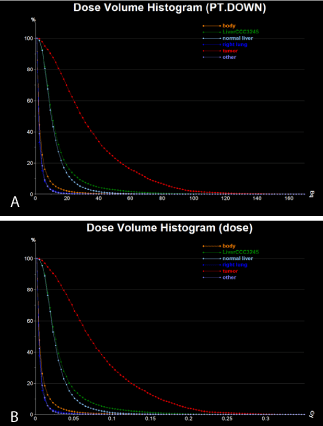
For dose map quantitative analysis, we used the VOI statistics
values (Table 1 and 2) and corresponding dose volume histograms
(DVH) (Figure 6).
The injected activity within the body contour has been 2.877x109
Bq. This originates a total intensity value of 2.0x106 (given by Stratos)
within the same body contour (Table 1). The calculated activity VOI
statistics, assuming adequate calibration, give origin to a tumor mean
dose of 0.082 a.u. (arbitrary units). For comparative purposes we may
assume that the a.u. are directly proportional to the total calculated
liver volume prescribed absorbed dose of 120 Gy. In this particular
case, the activity reaching the total liver was 2.791 GBq due to the
calculated hepato-pulmonary shunt (3% of the 2.877 GBq body
activity).
Body activity and tumor mean dose values were confirmed
by MATLAB® calculation based on the integral activity and dose
extracted from the normalized tumor (red) curve and respective dose
volume histograms (Figure 6). According to our calculations and
previously mentioned assumptions we need to implement a 1439 Bq/
intensity calibration factor in the TPS dose algorithm to calculate the
final activity and dose within the defined body contour.
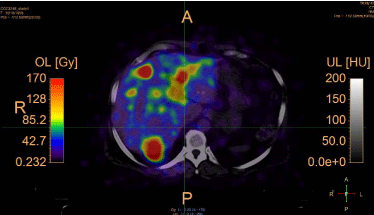
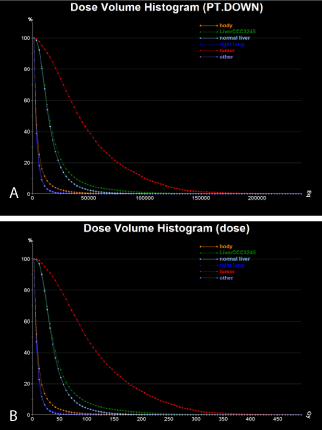
The calibrated absorbed dose distribution is shown in Figure 7
and the related statistics are shown in Table 3 and Table 4, and in
Figure 8.
The analysis of the VOI statistics of the calculated activity (Table
3), gives the total activity value for the body contour as 2.9x109 Bq in
agreement with the expected value of 2.877 GBq. The tumor mean
dose in the VOI statistics of the calculated absorbed dose (Table 4)
is 118 Gy, a deviation of -1.7% from the prescribed absorbed dose of
120 Gy.
Using the proposed calibration factor we would like to emphasize
that the calculated right lung Y90 activity (5.5x107 Bq, Table 3),
corresponds to a lung shunt value of 1.9%, one third less than the pretherapy
99mTc-MAA hepato-pulmonary shunt calculation.
The normal liver (91% of the total liver volume) mean dose (Table
4) was calculated as 40.4 Gy.
According to the presently accepted guidelines for stereotactic
body radiotherapy (SBRT) [10] 700 cm3 of normal liver must be
spared, i.e., the mean dose within this volume should be less than 15
Gy [11]. Based on the herein reported patient DVH (Figure 8B), the
normal liver calculated mean dose is 12 Gy in 700 cm3 corresponding
to 52% of total normal liver volume.
Figure 5
Table 1
Table 1
Initial VOI statistics of the calculated activity based on the pre-calibrated
Y90 PET images from the patient.
Table 2
Table 2
Initial VOI statistics of the calculated absorbed dose based on the precalibrated
Y90 PET images from the patient.
Figure 6
Figure 6
Initial DVH from the absorbed dose map calculated based on the
pre-calibrated Y90 PET images from the patient: (a) calculated activity, (b)
calculated absorbed dose.
Figure 7
Figure 7
Absorbed dose distribution calculated based on the calibrated Y90
PET images from the patient.
Table 3
Table 3
VOI statistics of the calculated activity based on the calibrated Y90 PET
images from the patient.
Table 4
Table 4
VOI statistics of the calculated absorbed dose based on the calibrated
Y90 PET images from the patient.
Figure 8
Figure 8
Final DVH from the absorbed dose map calculated based on the
calibrated Y90 PET images from the patient: (a) calculated activity (Bq), (b)
calculated absorbed dose (Gy).
Conclusion
This case report illustrates several safety parameters of Y90 (Therasphere®) RE liver therapy in a patient with multifocal liver metastases. The patient is clinically well and free of metabolic active and FDG avid liver disease sixteen months after RE liver therapy under imatinib. Using the dose calculation Stratos® (Imalytics, Philips®) algorithm software the entire tumor volume in the liver received 118 Gy, very close to the pre-therapy prescribed 120 Gy. In our case the normal liver dose was 12 Gy in 700 cm3 that is lower than the maximum recommended normal liver dose (< 15 Gy in 700 cm3).
Final Message
In order to improve the calculation algorithms under use we have
already ongoing work with the following main objectives:
1) to calculate calibration factors more appropriate to correct
for imaging data from SPECT, PET and CT;
2) to improve image co-registration between modalities;
3) to improve automatic segmentation algorithms;
4) to further evaluate the clinical “treatment planning” potential
of the dose calculation software algorithms used;
5) to implement Monte Carlo simulation software to improve
“treatment planning” dose calculation software algorithms.
Acknowledgment
We gratefully acknowledge the following people without whom all data would have not been possible to acquire, process and analyze: Alejandro Sosa Noreña, Marketing and Sales Manager of the Clinical Applications Research group at Philips; Professor Dr. Carlo Greco, Head of Radiation Oncology Department, CCU, FC for frequent critical motivation and incentive; Dr. Carlos Carvalho, Head of Digestive Tumor Department, CCU, FC for initial referral of the patient and continuous support; Nucliber/BTG – for partially financing research tests towards the calculation of calibration factors more appropriate for inter-modality imaging data correction; all technical staff at the Nuclear Medicine – Radiopharmacology Department, CCU, FC for their availability and dedication.
References
- Braat M, Samim M, Bosch M, Lam M. The role of 90Y-radioembolization in downstaging primary and secondary hepatic malignancies: a systematic review. Clin Transl Imaging. 2016; 1-13.
- Smits ML, Elschot M, Sze DY, Kao YH, Nijsen JF, Iagaru AH, et al. Radioembolization dosimetry: the road ahead. Cardiovasc Intervent Radiol. 2015; 38: 261-269.
- Garin E, Lenoir L, Rolland Y, Edeline J, Mesbah H, Laffont S, et al. Dosimetry Based on 99mTc-Macroaggregated Albumin SPECT/CT Accurately Predicts Tumor Response and Survival in Hepatocellular Carcinoma Patients Treated with 90Y-Loaded Glass Microspheres: Preliminary Results. J Nucl Med. 2012; 53: 255–263.
- Dieudonné A, Garin E, Laffont S, Rolland Y, Lebtahi R, Leguludec D, et al. Clinical feasibility of fast 3-dimensional dosimetry of the liver for treatment planning of hepatocellular carcinoma with 90Y-microspheres. J Nucl Med. 2011; 52: 1930-1937.
- Seza G, Manuel S, Jeffry S, Tatjana J, Michael S. Hepatic Structural Dosimetry in 90Y Microsphere Treatment: A Monte Carlo Modeling Approach Based on Lobular Microanatomy. J Nucl Med. 2010; 51: 301–310.
- Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: The MIRD equations for dose calculations. J Nucl Med. 2006; 47: 1209-1211.
- Giammarile F, Bodei L, Chiesa C, Flux G, Forrer F, Kraeber-Bodere F, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2011; 38: 1393-1406.
- Pasciak AS, Bourgeois AC, McKinney JM, Chang TT, Osborne DR, Acuff SN, et al. Radioembolization and the Dynamic Role of (90)Y PET/CT. Front Oncol. 2014; 4: 38.
- Paulus T, Fischer A, Loon P, Schweizer B, Gegenmantel E, Bippus R. IMALYTICS: The Philips translational and research workstation. MEDICAMUNDI. 2010; 54: 78-81.
- Grimm J, LaCouture T, Croce R, Yeo I, Zhu Y, Xue J. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011; 12: 3368.
- Scorsetti M, Clerici E, Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol. 2014; 5: 190-197.