Research Article
SOX2 Expression in Patients who Underwent Radical Cystectomy for Urothelial Carcinoma of the Bladder
Nottingham CU1, Patel SG2, Clark PE3, DeGraff DJ4, Gregg JR3, Anderson BB1, Paner GP5, Steinberg GD1 and Vander Griend DJ1,6*
1Department of Surgery, University of Chicago Medicine, USA
2Department of Urology, University of Oklahoma, USA
3Department of Urology, Vanderbilt University, USA
4Departments of Pathology, Pennsylvania State University College of Medicine, USA
5Department of Pathology, University of Chicago, USA
6The Ben May Institute for Cancer Research, University of Chicago, USA
*Corresponding author: Donald J. Vander Griend, Department of Surgery, University of Chicago, Section of Urology, 5841 S. Maryland Ave, MC6038, Chicago, IL 60637, USA
Published: 14 Jun, 2016
Cite this article as: Nottingham CU, Patel SG, Clark PE,
DeGraff DJ, Gregg JR, Anderson BB,
et al. SOX2 Expression in Patients who
Underwent Radical Cystectomy for
Urothelial Carcinoma of the Bladder.
Clin Oncol. 2016; 1: 1022.
Abstract
Background: SOX2 is a transcription factor essential for maintaining the survival and pluripotency of stem cells. Previous studies have described the role of SOX2 in breast, esophageal, and nonmuscle
invasive bladder cancer correlating with poor patient outcome.
Methods: We obtained a series of annotated TMAs from patients who underwent radical cystectomy
for urothelial carcinoma of the bladder (UCB). Tumor specimens were stained for SOX2 and scored
by a single genitourinary pathologist. Univariate and multivariate analyses were performed to
determine patient and pathologic characteristics correlating with SOX2 score. A univariate survival
analysis was performed using the Kaplan-Meier method and multivariate analysis with the Cox
proportional hazards model.
Results: From a total of 271 total patients who underwent radical cystectomy for UCB, 114 (42.1%)
had SOX2 scores >0. On univariate analysis SOX2 score >0 positively correlated with the presence
of carcinoma in situ (CIS) (p<0.002) and older patient age, but negatively correlated with pathologic
stage T2 and higher. On multivariate analysis older age and presence of CIS similarly correlated with
SOX2 score >0. There was no difference in overall survival between patients based on SOX2 score.
Conclusion: The presence of SOX2 in bladder cancer specimens correlated with the presence of CIS
at cystectomy, and patient age, but did not correlate with survival. Further investigation of early
high-grade lesions may elucidate the role of SOX2 in UCB carcinogenesis.
Keywords: SOX2; Bladder cancer; Carcinoma in situ; Cystectomy
Abbreviations
CIS: Carcinoma In Situ; SOX2: SRY Sex Determining Region Y-box 2; TMA: Tissue MicroArray; UCB: Urothelial Carcinoma of the Bladder; IHC: Immunohistochemistry
Introduction
The functional and clinical role of stem cell transcription factors in cancer remains an important
unresolved question. Expression of certain transcription factors maintain a pluripotent state
in embryonic stem cells [1,2]. The more commonly described factors include SOX2 [SRY (sex
determining region Y)-box 2], POU5F1 (formerly Oct4), and Nanog [1,2]. These transcription
factors inhibit expression of other genes that induce cellular differentiation [2], and their expression
can also induce a state of pluripotency in previously differentiated cells [3]. SOX2 has a well-described
role in the self-renewal of murine and human embryonic stem cells, and also provides a significant
contribution to the maintenance of stem cells in adult tissues of the nervous tissue and skin [4].
For this reason investigators have evaluated their role in carcinogenesis, tumor progression, and
therapy resistance. Prior studies have demonstrated upregulation of SOX2 in breast, brain, prostate,
and germ cell cancers, and especially in poorly-differentiated tumors [5,6]. This data suggests an
oncogenic role for SOX2 in certain cancers.
While SOX2 expression has been demonstrated in human bladder cancer, other stem cell
markers (Nanog and POU5F1) have not. Wezel et al. [7] demonstrated
no expression of OCT4A in both benign and malignant urothelium.
In a human UBC cell line, Ferreira-Teixeira et al. [8] observed fourfold
SOX2 mRNA increased expression over baseline, but noted that
expression of Nanog and POU5F1 mRNA was not increased over
baseline.
The role and expression pattern of SOX2 in urothelial carcinoma
of the bladder (UCB) has not been well defined. Bladder cancer is a
heterogeneous disease, with different histologic subtypes that each has
different biologic activity [9]. The most common histologic subtype of
UCB is urothelial cell carcinoma, for which there is both low and high
grade [9]. Amini et al. [10] demonstrated Sox2 expression in a human
UCB cell line (HT-1376). They also observed SOX2 expression in 9
of 10 human UCB specimens by RT-PCR, which included equivalent
representation from histologic grades I-III, and saw SOX2 expression
in all grade III specimens. Another study of over 100 patients with
non-muscle invasive UCB taken at transurethral resection suggested
that SOX2 expression correlated with poor recurrence free survival,
increased tumor size and number, and higher grade [11]. To date,
no studies have specifically evaluated the role of SOX2 in patients
requiring radical cystectomy. The objective of the present study was to
determine the expression pattern of SOX2 in human UCB specimens
at the time of radical cystectomy, and correlate its expression with
pathologic and oncologic outcomes. Based on previous studies in
both bladder and other cancers suggesting worse pathologic and
oncologic outcomes, we hypothesized that SOX2 expression would
positively correlate with advanced pathologic features at and worse
overall survival after cystectomy
Methods
Ethics statement
Human bladder tumor collection and tissue micro-array (TMA)
production was previously described in study from this series
of patients undergoing radical cystectomy [12]. Bladder tumor
specimens were collected by the Translational Pathology Shared
Resource, a core facility of the Vanderbilt-Ingram Cancer Center,
and later de-identified for testing and analysis. Clinical, pathological,
and follow-up data were collected via review of medical records and
extracted to the Research Electronic Data Capture database hosted at
Vanderbilt University. This project was approved by the Institutional
Review Board at Vanderbilt University.
Human tissue samples and generation of TMAs
TMAs were generated using bladder tissue cores from 271
patients who underwent radical cystectomy, bilateral pelvic
lymphadenectomy, and urinary diversion for urothelial or squamous
carcinoma of the bladder between January 2000 and May 2010.
Hematoxylin and eosin (H&E) slides from the TMAs were prepared.
Patients were subsequently followed up at 3 months, 6 months, and
then at increasing intervals based on individual surgeons' practice
patterns by physical examination, laboratory studies, and both chest
and abdominal imaging. Clinico-pathologic data were collected and
included patient demographics, such as age at time of surgery, sex,
and comorbidity recorded as age-adjusted Charlson comorbidity
index [13], and tumor characteristics, such as AJCC tumor stage [14],
and grade. The original H&E slides were reviewed and diagnostic
tissue was marked for construction of a TMA using a manual arrayer
(Beecher Instruments, Sun Prairie, WI). One to three tissue cores
(each 1.5 mm) of representative areas from each of the selected
formalin-fixed, paraffin-embedded (FFPE) UBC tissue blocks were
sampled to generate each TMA. The histopathological diagnosis
of tissue samples represented on the TMA were identified for each
patient (wherever available), including adjacent benign urothelium,
noninvasive papillary urothelial carcinoma (pTa), urothelial
carcinoma in situ (pTis, also referred to as CIS), or invasive urothelial
carcinoma (pT1-pT4), and were recorded in a Research Electronic
Data Capture relational database.
IHC staining and evaluation
Immunohistochemistry staining (IHC) for SOX2 (D6D9 XP,
Cell Signaling Technology; rabbit monoclonal) was performed on
FFPE sections managed by the University of Chicago Human Tissue
Resource Core facility. After deparaffinization and rehydration,
tissues were treated with antigen retrieval buffer (S1699 from DAKO;
Glostrup, Denmark) in a steamer for 20 minutes. Anti- SOX2
antibody (1:25 dilution) was applied for 1 hour at room temperature
in a humidity chamber. Following TBS wash, the antigen-antibody
binding was detected with Envision+system (DAKO, K4001 for mouse
primary antibodies) and DAB+Chromogen (DAKO, K3468). Tissue
sections were briefly immersed in hematoxylin for counterstaining
and were cover-slipped.
Tissues were analyzed by a trained genitourinary pathologist
and scored on percentage of cells with positive nuclear staining (0
= no staining; 1 = 1–10% positive cells; 2 = 11–50% positive cells;
and 3 = 50-100% positive cells), as well as the intensity of staining (0
= no staining; 1 = weak staining; 2 = moderate staining; 3 = strong
staining). The two scores were multiplied to create a composite SOX2
score. The composite score was added to the Research Electronic
Data Capture relational database. Patients were then divided into two
groups for analysis: 1) patients with a SOX2 score of 0, and 2) patients
with a SOX2 score > 0. Patients with a composite score of 0 were
considered SOX2 negative, whereas patients with a score > 0 were
considered SOX2 positive. For images, slides were digitized using a
Pannoramic Scan Whole Slide Scanner (Cambridge Research and
Instrumentation; Hopkinton, MA) and images captured using the
Pannoramic Viewer software version 1.14.50 (3DHistech; Budapest,
Hungary).
Statistical analysis
Categorical variables between SOX2 negative and positive groups
were compared using Pearson's Chi-squared tests. Univariate and
multivariate logistic regression was used to compare likelihood of
SOX2 positive staining when controlling for patient demographic
factors. A Kaplan-Meier analysis was used to describe the mean,
3-year, and 5-year survival for patients in both groups, and to
compare them for a difference in survival. Multivariate predictors
for survival were evaluated with a Cox proportional hazards model.
Stata version 14.0 (Stata Corp., College Station, TX) was used for all
statistical analysis.
Results
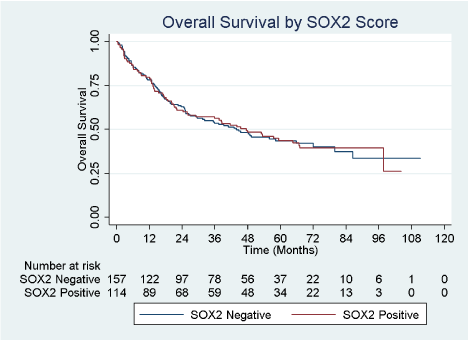
From a total of 271 total patients who underwent radical cystectomy for UBC, 114 (42.1%) had SOX2 scores > 0. Representative images of SOX2-positive and SOX2-negative tumors are shown in (Figure 1). Patient demographics are described in Table 1 with patients separated by SOX2 composite score groups into those whose tumor specimens stained negative versus positive for SOX2. The study cohort had a similar distribution of patients by baseline clinical characteristics of age, sex, race, and Charleson comorbidity score (all p > 0.05). Pathologic characteristics of primary cancer cell type, clinical tumor stage, pathologic tumor stage, tumor grade, nodal status, proportion of patients upstaged at cystectomy, and proportion of patients who received any intravesical therapy prior to cystectomy were also similar (all p > 0.05). However, a significantly higher proportion of SOX2 positive patients had CIS present at cystectomy (66.4% vs. 44.2% p < 0.001). We then sought to identify predictors of SOX2 positivity by performing a univariable analysis to evaluate age, sex, race, Charleson score, presence of non-urothelial histology, clinical stage prior to cystectomy, CIS at final pathology, final pathologic tumor stage, final pathologic grade, presence of lymph node metastases, upstaging at cystectomy, and receipt of any intravesical treatment prior to cystectomy. Patients of age groups 60 – 69 years (OR 3.87; 95% CI 1.20 – 12.46; p=0.023), 70 – 79 years (OR 3.21; 95% CI 1.00 – 10.27; p=0.050), and ≥ 80 years (OR 5.19; 95% CI 1.28 – 21.08; p=0.021) had a higher odds of SOX2 score > 0 compared to referent age < 50 years, while patients aged 50 – 59 years did not. Patients with CIS at cystectomy also had a higher odds of having a SOX2 score > 0 (OR 2.50; 95% CI 1.51 – 4.13; p<0.001). By contrast, patients with pathologic stage T2 (OR 0.53; 95% CI 0.28 – 0.99; p=0.046), T3 (OR 0.49; 95% CI 0.26 – 0.94; p=0.031), and T4 (OR 0.41; 95% CI 0.18 – 0.97; p=0.044) all had significantly lower odds of a SOX2 score > 0 compared to patients with pathologic stage ≤ T1. Table 2 shows a multivariate logistic regression model of correlation with SOX2 positivity. When controlling for sex, race, Charleson comorbidity score, pathologic tumor stage, tumor grade, nodal status, and prior receipt of intravesical therapy, we observed that patient age 60 years and older (all p < 0.05) as well as the presence of CIS at cystectomy (OR 2.39, 95% CI 1.37 – 4.17, p = 0.002) were both still positively correlated with patients having a Sox-2 score > 0. While there was a trend towards SOX2 positivity in higher pathologic stages, this did not reach significance. When separately analyzing the pathologic groups as bladder-confined (pTaN0, pTisN0, pT1N0, and pT2N0) versus locally-advanced (pT3N0 and pT4N0) and nodepositive (pT any), there was a similar trend towards SOX2 positivity in the locally-advanced group (p=0.052) but not in the node-positive group (p=0.901) (data not shown). There was no difference in overall survival between patients who were SOX2 negative and SOX2 positive (Figure 2). Median, 3-year, and 5-year survival for SOX2 negative patients were 43.8 months, 53.6%, and 43.5%, respectively, and for SOX2 positive patients were 45.5 months, 56.3%, and 43.7%, respectively (Table 3). Using a multivariate Cox Proportional Hazards model controlling for sex, race, and Charleson Score, we observed that age ≥ 80 years, higher pathologic tumor stages (pT2 and higher), and lymph node metastasis were correlated with shorter survival (Table 4). The presence of SOX2 and CIS at cystectomy did not correlate with survival
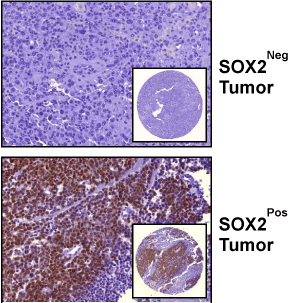
Figure 1
Figure 1
Immunohistochemical Detection of SOX2 in Urothelial Tumors.
Images represent tumors with a SOX2 score of zero (upper image), and a
SOX2 score >0 (lower panel). Images are at a 40x magnification; and inset
images are of TMA spot at a 5x magnification.
Table 1
Table 2
Figure 2
Table 3
Discussion
Our understanding of the role of stem cell associated transcription
factors in UCB remains in its infancy, particularly in regard to SOX2
biology and its correlation with clinical outcomes. Few studies have
evaluated the expression profile of SOX2 in clinical UCB specimens
[10,11]. We report the first description of SOX2 expression in UCB in
a radical cystectomy cohort, and observed that it correlated with the
presence of CIS at the time of cystectomy as well as with older patient
age. The pattern of SOX2 expression was somewhat unexpected
given its previously-described profile in both laboratory and clinical
specimens. While the majority of all UCB patients have low-grade
non-invasive disease that will be managed endoscopically and have a
low risk if any of progression to high-grade disease [15], our patients
mostly have high-grade that will inevitably metastasize if untreated.
Patients with high-grade disease that is not invasive to the muscularis
propria are typically treated with intravesical BCG immunotherapy
[15], but patients with BCG-refractory or muscle-invasive disease
are typically managed with radical cystectomy or chemotherapy
with radiation. The patients in this study had aggressive high-grade
disease that have failed BCG therapy or otherwise had advanced
pathology requiring cystectomy. The prior studies demonstrating
SOX2 expression in high grade lesions [10] and SOX2 correlation
with adverse pathologic and clinical outcomes [11] suggest that it
would both be highly expressed and positively correlate with poor
outcomes in patients requiring radical cystectomy. However, we
observed SOX2 expression in 42.1% of the present study population
and found no correlation with advanced pathologic stage, metastatic
disease, or overall survival. In the study by Ruan et al. [11], they
observed that 53% of 126 bladder cancer specimens (all pathologic
stage T1 taken at time of transurethral resection of bladder tumor)
had high levels of staining for SOX2, which is similar to our observed
percentage although somewhat higher. This difference in SOX2
expression between our groups raises the question of whether SOX2
expression changes as the cancer advances in stage. The reason for a
strong correlation between CIS at the time of cystectomy and SOX2
positivity is not readily apparent in this data. In the time course
of progression from carcinogenesis to muscle invasion, the CIS
stage temporally occurs closer to the initiation of carcinogenesis.
CIS is a high-grade lesion that has the same genetic abnormalities
as high-grade muscle-invasive disease [16], but has simply been
observed before this progression has occurred. While previous
data has suggested that SOX2 promotes pluripotency and therefore
may initiate carcinogenesis, the data in this study suggests SOX2
expression may decrease as the bladder cancer progresses in stage.
Our univariate analysis revealed an inverse correlation with SOX2
expression and pathologic tumor stage of T2 or higher, although
on multivariate analysis this trend was present but did not reach
statistical significance.
In other malignancies such as colorectal cancer, high SOX2
expression correlates with positive nodal metastasis, liver metastasis,
and WHO grade [17]. SOX2 is also more highly expressed in laryngeal
and esophageal cancer, and similarly correlates with worse clinical
outcomes [18,19]. SOX2 has been proposed as a lineage-survival
oncogene in squamous cancers of the lung and esophagus, by which
it promotes immortalization of these cells from the normal state [20].
In these malignancies, SOX2 expression may remain present and
contribute to the progression of the cancer. Our study, alternatively,
suggests that SOX2 may be present at the inception of the UCB, but
may not remain as it progresses. This may represent an intrinsic
difference in the biology of the these types of cancer. In non-cancerous
nervous tissue responding to injury, glial cells that normally maintain
SOX2 (and their pluripotency) reduce SOX2 expression as they
differentiate into other more mature cells to remyelinate neurons.
Because SOX2 regulates multiple upstream genes [2,4], the different
cell populations may upregulate or downregulate SOX2 for variable
phenotypic expressions. SOX2 as a transcription factor facilitates the
expression of multiple downstream genes [4,5], and the sets of genes
may vary depending on the cell type. The types of genes regulated
by SOX2 may be variable in malignancies of different organ sites,
as well. The potential mechanism for SOX2 upregulation in UCB is
unclear. Islam et al demonstrated in a murine xenograft model that
TGF-β1 induced sonic hedgehog signaling, which led to upregulation
of stem cell markers including SOX2. Data from The Cancer Genome
Atlas Research Network [21] identified 32 recurrent mutations from
131 UCB cases, but SOX2 was not one of them. No prior studies
including the present have suggested a mutation in SOX2, so further
investigation into the potential interaction between any known
mutated genes and pluripotent stem cell genes may provide further
insight as to the full biologic spectrum of this disease. This study also
found a positive association between higher age and SOX2 positivity,
which was unexpected. In the aforementioned study of patients with
pathologic stage T1 bladder cancer, the authors found no difference
in SOX2 expression between patients above versus below 65 years of
age [11]. A correlation between SOX2 expression and age in other
malignancies has not always been observed [18,19]. however, a study
of patients with squamous cell lung cancer actually showed increased
SOX2 expression correlating with younger patient age [22], which was
opposite to our study, and for which the authors were unclear of the
etiology. This one found no correlation between overall survival and
SOX2 expression, and did not report a correlation between overall
survival and age. This study does have limitations, one of which being
the retrospective nature of data analysis. One such concern is the
accuracy of the data sample, although many elements of the data are
congruous with our current knowledge of bladder cancer; for example,
this sample replicated prior studies showing higher pathologic stage,
node-positive metastatic disease, and patients aged ≥ 80 years have
lower overall survival. Another limitation is that we do not have
certain elements of data such as what specific intravesical therapies
patients received and how long they had received them. Vanderbilt
is a tertiary referral center so many of these patients received bladder
cancer treatment regimens from different providers using different
techniques particularly with regard to intravesical therapy regimens,
which are not entirely standard [23] prior to referral. This allows for
some heterogeneity in the stage of patients who undergo cystectomy.
A third limitation is that TMAs represent a sampling of the tumor but
may not entirely represent the entire bladder tumor specimen, which
could potentially be heterogeneous with SOX2 expression.
Table 4
Conclusion
SOX2 expression in clinical muscle-invasive UCB did not correlate with overall patient survival, but was highly expressed in tumor specimens with CIS and in older patients. Further investigation into the role of SOX2 and other pluripotency-mediating transcription factors in early stage UCB may provide insight into urothelial carcinogenesis.
References
- Young RA. Control of the embryonic stem cell state. Cell. 2011; 144: 940- 954.
- Seymour T, Twigger A, Kakulas F. Pluripotency Genes and Their Functions in the Normal and Aberrant Breast and Brain. Int J Mol Sci. 2015; 16: 27288-27301.
- Moad M, Pal D, Hepburn AC, Williamson SC, Wilson L, Lako M, et al. A Novel Model of Urinary Tract Differentiation, Tissue Regeneration, and Disease: Reprogramming Human Prostate and Bladder Cells into Induced Pluripotent Stem Cells. Eur Urol. 2013; 64: 753–761.
- Feng R, Wen J. Overview of the roles of Sox2 in stem cell and development. Biol Chem. 2015; 396: 883-891.
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008; 40: 499-507.
- Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS One. 2013; 8: e53701.
- Wezel F, Pearson J, Kirkwood LA, Southgate J. Differential expression of Oct4 variants and pseudogenes in normal urothelium and urothelial cancer. Am J Pathol. 2013; 183: 1128-1136.
- Ferreira-Teixeira M, Parada B, Rodrigues-Santos P, Alves V, Ramalho JS, Caramelo F, et al. Functional and molecular characterization of cancer stem-like cells in bladder cancer: a potential signature for muscle-invasive tumors. Oncotarget. 2015; 6: 36185–36201.
- Black PC, Brown GA, Dinney CP. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol. 2009; 27: 3-7.
- Amini S, Fathi F, Mobalegi J, Sofimajidpour H, Ghadimi T. The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines. Anat Cell Biol. 2014; 47: 1-11.
- Ruan J, Wei B, Xu Z, Yang S, Zhou Y, Yu M, et al. Predictive value of Sox2 expression in transurethral resection specimens in patients with T1 bladder cancer. Med Oncol. 2013; 30: 445.
- Reddy OL, Cates JM, Gellert LL, Crist HS, Yang Z, Yamashita H, et al. Loss of FOXA1 Drives Sexually Dimorphic Changes in Urothelial Differentiation and Is an Independent Predictor of Poor Prognosis in Bladder Cancer. Am J Pathol. 2015; 185: 1385-1395.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994; 47: 1245-1251.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17: 1471-1474.
- Kamat AM, Witjes JA, Brausi M, Soloway M, Lamm D, Persad R, et al. Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. J Urol. 2014; 192: 305-315.
- Höglund M. The bladder cancer genome; chromosomal changes as prognostic makers, opportunities, and obstacles. Urol Oncol. 2012; 30: 533-540.
- Neumann J, Bahr F, Horst D, Kriegl L, Engel J, Luque RM, et al. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer. 2011; 11: 518.
- Tang XB, Shen XH, Li L, Zhang YF, Chen GQ. SOX2 overexpression correlates with poor prognosis in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2013; 40: 481-486.
- Wang Q, He W, Lu C, Wang Z, Wang J, Giercksky KE, et al. Oct3/4 and Sox2 Are Significantly Associated with an Unfavorable Clinical Outcome in Human Esophageal Squamous Cell Carcinoma. Anticancer Res. 2009; 29: 1233–1241.
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009; 41: 1238-1242.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014; 507: 315- 322.
- Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, Maier S, et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol. 2011; 24: 944- 953.
- Witjes JA, Palou J, Soloway M, Lamm D, Kamat AM, Brausi M, et al. Current clinical practice gaps in the treatment of intermediate- and highrisk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guérin (BCG): results of an international individual patient data survey (IPDS). BJU Int. 2013; 112: 742–750.