Technical Note
In Vivo Diagnosis of Skin Cancer through Reflectance Confocal Microscopy
Grant-Kels JM1*, Rabinovitz HS2, Oliviero M2 and Scope A3*
1Department of Dermatology, University of Connecticut Health Center, USA
2Department of Dermatology, University of Miami Miller School of Medicine, USA
3Department of Dermatology, Sheba Medical Center and Sackler Faculty of Medicine, Tel Aviv University, Israel
*Corresponding author: Grant-Kels JM, Department of Dermatology, University of Connecticut Health Center, 263 Farmington Avenue, MC 6230, Farmngton, CT 06030, USA
Published: 18 Jun, 2016
Cite this article as: Grant-Kels JM, Rabinovitz HS, Oliviero
M, Scope A. In Vivo Diagnosis of Skin
Cancer through Reflectance Confocal
Microscopy. Clin Oncol. 2016; 1: 1021.
Introduction
In vivo reflectance confocal microscopy (RCM) is an FDA approved non-invasive optical
imaging technique that provides high-resolution cellular images of the skin. This new technology
assists the dermatologist in the early diagnosis of skin cancers, thereby reducing the number of
unnecessary biopsies performed and improving the malignant to benign biopsy ratio. In 2016, this
new technology was awarded a CPT (Current Procedural Terminology) code by the American
Medical Association; clinicians can now offer an RCM examination to their patients, while being
reimbursed for their effort.
Briefly, RCM is based on a low-power laser, which emits near-infrared light that is projected
through a lens system into the skin. The light is backscattered by microscopic tissue components in
the epidermis and dermis (e.g., melanin and keratin are strong back-reflectors of RCM light). Only
light that returns from a small focal point in the skin will enter the RCM detector through a gating
pinhole; all light from outside the desired focal point will be rejected. The RCM instantaneously
scans a 500µmX500µm area of tissue in the horizontal plane to produce an en face high-resolution
image of tissue (termed an "optical section"). The images appear in black and white – tissue
structures that backscatter light will appear bright, while the background skin will appear dark. The
resulting optical section is comparable to high magnification (about 30x) histopathology, except
that the optical "tissue slides" section the skin parallel to the skin's surface, appear in black and white,
and are obtained in vivo completely non-invasively. RCM provides cellular detail of the epidermis
and papillary dermis down to a depth of about 200μm. The RCM device also incorporates a software
that stitches individual optical sections into mosaic images, allowing a field of view of up to 8X8mm
(akin to 3-4x low-magnification histopathology). Additionally, RCM has an automated "stacking"
function, which allows acquisition of sequential individual optical sections at a particular site in the
lesion from the stratum corneum to the papillary dermis.
Previous articles and book chapters have outlined the features seen with RCM imaging
of normal skin [1-3]. The stratum corneum appears as a highly refractive surface with visible
furrows representing the skin folds. The stratum granulosum and stratum spinosum demonstrate
a "honeycombed" pattern, with regular spacing between the keratinocytes and bright cytoplasm
and dark oval nuclei. Keratinocytes are noted to diminish in size (from 35 μm to 15 μm) as they
approach the basal cell layer. The basal layer appears as aggregates of bright cells corresponding to
the horizontal sectioning at suprapapillary plates (described as "cobblestone") or in a circular pattern
(termed "edged papillae") around a dark papillary dermis corresponding to horizontal sectioning of
dermal papillae. The papillary dermis shows fibrillary collagen bundles and blood vessels, in which
blood cells can be seen coursing through the vessels, during real-time imaging.
Images can be read immediately at the bedside and an RCM diagnosis can be rendered to the
patient in real-time. Alternatively, RCM images may be transmitted via telemedicine (tele-RCM),
using a store-and-forward technique, to an expert who can interpret the images and produce a
structured report, similar to a dermatopathology report [4]. The expert reader may receive patient
demographic and anamnestic information, a digital dermatoscopic image of the lesion, and a set of
RCM images including three to four mosaic maps of the suprabasal epidermis, basal epidermis /
dermal-epidermal junction (DEJ), and papillary dermis, as well as image stacks at various foci of the
lesion. As in all telemedicine scenarios, a secure server that safeguards information with DICOM
(Digital Imaging and Communications in Medicine) and HIPAA (Health Insurance Portability and
Accountability Act) compliant security in the United States is required. Utilizing a cloud-based
server and high-speed internet connection (100 mbps), the expert
reader can view the images on a high-resolution computer monitor,
a tablet, or even mobile phone. Appropriate software affords the
reader the ability to navigate through the images, zooming in and out,
reminiscent of the functionality of a light microsocpe.
A meta-analysis of the diagnostic accuracy of RCM for melanoma
identified a sensitivity of 93% and a specificity 76% [5]. When is it
appropriate to apply RCM? RCM is currently best used for the
evaluation of pigmented (whereby the source of pigment is melanin,
e.g., melanocytic neoplasms) or keratinocytic lesions (e.g. basal cell
carcinoma and squamous cell carcinoma). RCM can be used in lieu
of an initial diagnostic shave biopsy for lesions that are clinically
or dermoscopically equivocal for diagnosis; for example, a patient
may be reluctant to have a superfluous biopsy done in cosmetically
sensitive areas like the face or in sites that are prone to hypertrophic
scars. A clear-cut RCM finding of skin cancer will increase the
clinicians' confidence in the required management, and will likely
convince the patient to proceed to surgical therapy. On the other
hand, an unnecessary biopsy and scarring will be saved if the lesion
proves to be benign. At our institutions, we have found RCM to be
also useful in patients with many atypical nevi. RCM has been shown
to reduce the number of biopsies we need to perform by about 50%,
resulting in an improved malignant-to-benign ratio in the biopsies we
do perform. Indeed, studies have demonstrated that RCM was able to
greatly reduce the number of lesions needed to excise to identify one
skin cancer [6,7]. Finally, we have also used RCM to non-invasively
monitor the response of skin cancer (e.g., facial lentigo maligna) to
imiquimod or other topical chemotherapeutic agents [8].
Figure 1
Figure 2
Figure 2
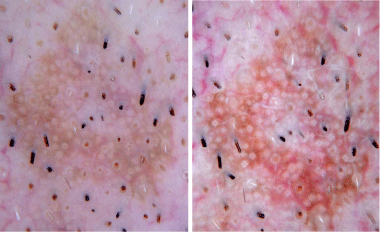
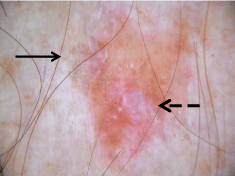
Contact non-polarized (left) and contact polarized (right)
dermoscopy revealing white circles and keratotic plugs.
Figure 3
Figure 3
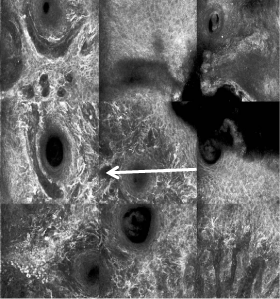
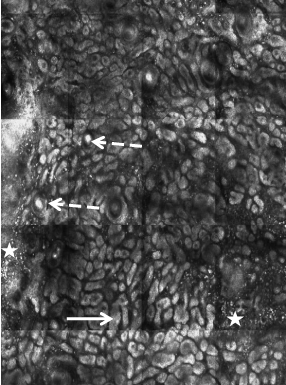
RCM revealing dendritic melanocytes at the epidermis and
extending down follicular infundibula.
Clinical Example of Use of RCM in Clinical Practice
Case 1
A 54-year-old man with a past medical history of facial melanoma
on sun-damaged skin noticed a new enlarging pigmented lesion on
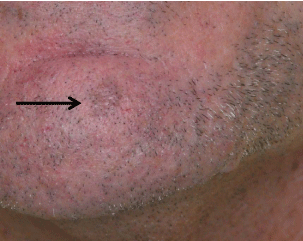
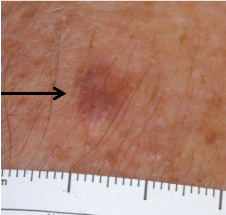
his chin. Examination showed a 6 mm tan macule (Figure 1), and
dermoscopy demonstrated white circles and keratotic plugs (Figure2).
The patient was very concerned about scarring at this site. Based
upon dermoscopy, the dermatologist considered Bowen's disease as a
probable diagnosis and hence contemplated treating the lesion with
5% Fluorouracil (5-FU) topically. To secure the diagnosis prior to
topical therapy, RCM was performed. However, RCM revealed many
large, bright dendritic cells (compatible with atypical melanocytes)
within the suprabasal and basal epidermis and extending down the
follicular infundibula (Figure 3); the RCM findings were consistent
with the diagnosis of melanoma on sun-damaged skin. An excision
demonstrated atypical melanocytes within the epidermis, along
the DEJ and extending down follicular infundibula, confirming the
diagnosis of melanoma in situ (Figure 4). By using RCM in this case,
we were able to confidently proceed directly to a definitive excision
without performing an initial shave biopsy, as well as to avoid treating
the patient inappropriately with topical 5-FU.
Case 2
A 66-year-old man with a history of melanoma and non
melanoma skin cancers developed a new pink brown 6 mm papule of
the right arm (Figure 5). The clinical differential diagnosis included an
atypical melanocytic nevus, melanoma, lichen planus- like keratosis
(LPLK), or a pigmented SCC. Dermoscopically (Figure 6), the lesion
exhibited focal gray-dot granules (suggestive of melanophages in
the papillary dermis) and shiny white structures (also known as
the chrysalis sign, suggestive of superficial dermal fibrosis). RCM
imaging at the DEJ level demonstrated elongated cords with bulbous
tips of the epidermis, as well as cornal cysts (Figure 7). There were
some bright plump-bright cells and bright dots (compatible with an
inflammatory cell infiltrate) seen within the superficial dermis. These
findings confirmed the diagnosis of a lichen planus-like keratosis.
The patient was reassured the lesion is benign and that no biopsy is
necessary, sparing him discomfort and unnecessary scarring.
Figure 4
Figure 4
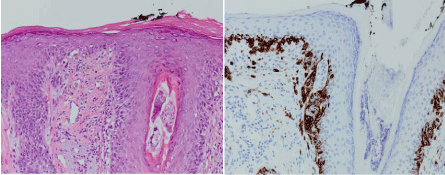
Histopathology demonstrating a typical melanocytes at the dermalepidermal
junction with hematoxylin and eosin staining (left) and confirmed
with HMB-45 immunoperoxidase staining (right) consistent with melanoma in
situ on sun-damaged skin.
Figure 5
Figure 6
Figure 6
Dermoscopy demonstrated focal gray granularity (arrow) and a
shiny white area (broken arrow).
Figure 7
Figure 7
RCM demonstrates elongated cords with bulbous tips (white arrow)
and corneal cysts (dashed white arrow) in association with bright plumpbright
cells and bright dots (star). These are RCM features of lichen planuslike
keratosis.
References
- Que K, Fraga-Braghiroli N, Grant-Kels JM, Rabinovitz H, Oliviero M, Scope A. Through the looking glass: A glimpse at reflectance confocal microscopy and its potential clinical applications in dermatology and dermatologic surgery. J Am Acad Dermatol. 2015; 73: 276-284.
- Scope A, Benvenuto-Andrade C, Agero AL, Malvehy J, Puig S, Rajadhyaksha M, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007; 57: 644-658.
- Lieb JA, Gill M, Patel YG, Rajadhyaksha M, Gonzalez S. Normal skin. In: Gonzalez S, Gill M, Halpern AC, editors. Reflectance confocal microscopy of cutaneous tumors. London: Informa Healthcare. 2008; 7-29.
- Witkowski A, Ludzik J, Soyer P. Telediagnosis with confocal microscopy: a reality or a dream? Dermatology Clinics.
- Stevenson AD, Mickan S, Mallett S, Ayya M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanomadiagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013; 3: 19-27.
- Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014; 170: 802-808.
- Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014; 171: 1044-1051.
- Nadiminti H, Scope A, Marghoob AA, Busam K, Nehal KS. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010; 36: 177-184.